-
 GEOLOGY & GEOPHYSICS
G&G
| SARAH LAMBART
GEOLOGY & GEOPHYSICS
G&G
| SARAH LAMBART
-
 GEOLOGY & GEOPHYSICS | SARAH LAMBART
GEOLOGY & GEOPHYSICS | SARAH LAMBART
- Pictures
- Links
- Resources
- Publications
- MagMaX Lab

Publications:
+ Student
Asmaa Boujibar, Kevin Righter, Emmanuel Fontaine, Max Collinet, Sarah Lambart, Larry R. Nittler, Kellye M. Pando, Lisa R. Dianelson, 2025. A Pyroxenite Mantle on Mercury? Experimental Insights from Enstatite Chondrite Melting at Pressures up to 5 GPa. Icarus, 437, 116602. doi:10.1016/j.icarus.2025.116602
Rong Xu, Yue Cai, Sarah Lambart, Chunfei Chen, Jun-Bo Zhang, Mei-Fu Zhou, Jia Liu, Zhongjie Bai, Tao Wu, Feng Huang, Ting Ruan, Yongsheng Liu 2025. Heavy boron isotopes in intraplate basalts reveal recycled carbonate in the mantle, Science Advances, 11, eads5104. doi:10.1126/sciadv.ads5104
Lynne J. Elkins, Sarah Lambart, 2024. Uranium-series disequilibria in MORB, revisited: A systematic numerical approach to partial melting of a heterogeneous mantle, Volcanica, 7(2), 685-715. doi:10.30909/vol.07.02.685715
Susana Henriquez, Gerel Ochir, Sarah Lambart, Cari L. Johnson, Laura E. Webb, Peter C. Lippert, 2024. From an accretionary margin to a sediment-rich collision: Spatiotemporal evolution of the magmatism during the closure of the Mongol-Okhotsk Ocean, Gondwana Research, 135, 180-197. doi:10.1016/j.gr.2024.07.015
Ashley M. Morris+, Sarah Lambart, Michael A. Stearns, John R. Bowman, Morgan T. Jones, Geoffroy T.F. Mohn, Graham Andrews, John Millet, Christian Tegner, Sayantani Chatterjee, Joost Frieling, Pengyuan Guo, David W. Jolley, Emily H. Cunningham+, Christian Berndt, Sverre Planke, Carlos A. Alvarez Zarikian, Peter Betlem, Henk Brinkhuis, Marialena Christopoulou, Eric Ferré, Irina Y. Filina, Dustin Harper, Jack Longman, Reed P. Scherer, Natalia Varela, Weimu Xu, Stacy L. Yager, Amar Agarwal, Vincent J. Clementi, 2024. Evidence for Low-pressure Crustal Anatexis During the Northeast Atlantic Break-up, Geochemistry, Geophysics, Geosystems, 25(7), e2023GC011413. doi:10.1029/2023GC011413
Madeleine L. Vickers, Morgan T. Jones, Jack Longman, David Evans, Clemens V. Ullmann, Ella Wulfsberg Stokke, Martin Vickers, Joost Frieling, Dustin T. Harper, Vincent J. Clementi, and IODP Expedition 396 Scientists, 2024. Paleocene–Eocene age glendonites from the Mid-Norwegian Margin – indicators of cold snaps in the hothouse?, Climate of the Past, 20, 1-23. doi:10.5194/cp-20-1-2024
Xu R., Lambart S., Li M., Nebel O., Bai Z., Zhang J., Zhang G., Goa J., Zhong H., Liu Y., 2024. Iron isotope evidence on continental intraplate basalts for mantle lithosphere imprint on heterogenous asthenospheric melts, Earth and Planetary Science Letters, 625, 118499. doi:10.1016/j.epsl.2023.118499
Christian Berndt, Sverre Planke, Carlos A. Alvarez Zarikian, Joost Frieling, Morgan T. Jones, John M. Millett, Henk Brinkhuis, Stefan Bünz, Henrik H. Svensen, Jack Longman, Reed P. Scherer, Jens Karstens, Ben Manton, Mei Nelissen, Brandon Reed, Jan Inge Faleide, Ritske S. Huismans, Amar Agarwal, Graham D. M. Andrews, Peter Betlem, Joyeeta Bhattacharya, Sayantani Chatterjee, Marialena Christopoulou, Vincent J. Clementi, Eric C. Ferré, Irina Y. Filina, Pengyuan Guo, Dustin T. Harper, Sarah Lambart, Geoffroy Mohn, Reina Nakaoka, Christian Tegner, Natalia Varela, Mengyuan Wang, Weimu Xu & Stacy L. Yager, 2023. Shallow-water hydrothermal venting linked to the Palaeocene–Eocene Thermal Maximum, Nature Geoscience. doi:10.1038/s41561-023-01246-8
Lynne J. Elkins, Bernard Bourdon, Sarah Lambart, 2023. Corrigendum to “Testing pyroxenite versus peridotite sources for marine basalts using U-series isotopes” [Lithos 332–333 (2019) 226–244].Lithos. 452-453, 107217. doi:10.1016/j.lithos.2023.107217
Marthe Klöcking, Lesley Wyborn, Kerstin A. Lehnert, Bryant Ware, Alexander M. Prent, Lucia Profeta, Fabian Kohlmann, Wayne Noble, Ian Bruno, Sarah Lambart, Halimulati Ananuer, Nicholas D. Barber, Harry Becker, Maurice Brodbeck, Hang Deng, Kai Deng, Kirsten Elger, ..., Tengfei Zhou, 2023. Community recommendations for geochemical data, services and analytical capabilities in the 21st century, Geochimica et Cosmochimica Acta, 351, 192-205 doi:10.1016/j.gca.2023.04.024
Otto I. Lang+, Sarah Lambart 2022. First-row transition elements in pyroxenites and peridotites: A promising tool for constraining mantle source mineralogy, Chemical Geology, 121137. doi:10.1016/j.chemgeo.2022.121137
Adrien J. Mourey, Thomas Shea, Kendra J. Lynn, Allan H. Lerner, Sarah Lambart, Fidel Costa, Jeffrey Oalmann, R. Lopaka Lee, Cheryl Gansecki 2022. Trace elements in olivine fingerprint the source of 2018 magmas and shed light on explosive-effusive eruption cycles at Kılauea Volcano, Earth and Planetary Science Letters, 595, 117769. doi:10.1016/j.epsl.2022.117769
Juliette Pin, Lyderic France, Sarah Lambart, Laurie Reisberg, 2022. Thermodynamic modeling of melt addition to peridotite: implications for the refertilization of the non-cratonic continental mantle lithosphere., Chemical Geology, 609, 121050. doi:10.1016/j.chemgeo.2022.121050
Sarah Lambart, Sarah Hamilton+, Otto I. Lang+, 2022. Compositional variability of San Carlos Olivine, Chemical Geology, 605, 120968. doi:10.1016/j.chemgeo.2022.120968
Rong Xu, Yongsheng Liu, Sarah Lambart, Kaj Hoernle, Yangtao Zhu, Zongqi Zou, Junbo Zhang, Zaicong Wang, Ming Li, Frederic Moynier, Keqing Zong, Haihong Chen, Zhaochu Hu, 2022. Decoupled Zn-Sr-Nd isotopic composition of continental intraplate basalts caused by two-stage melting process, Geochimica et Cosmochimica Acta, 326, 234-252. doi:10.1016/j.gca.2022.03.014
Ananya Mallik, Sarah Lambart, Emily Chin, 2021. Tracking the evolution of magmas from heterogeneous mantle sources to eruption. In: Konter J., Ballmer M, Cottaar S, & Marquardt H. (Eds.), Mantle Convection and Surface Expressions, Geophysical Monograph 263, invited contribution. doi:10.1002/9781119528609.ch6
Rong Xu, Yongsheng Liu, Sarah Lambart, 2020. Melting of a hydrous peridotite mantle source under the Emeishan large igneous province. Earth Science Reviews. doi:10.1016/j.earscirev.2020.103253
Sarah Lambart, Janne M. Koornneef, Marc-Alban Millet, Gareth R. Davies, Matthew Cook & C. Johan Lissenberg 2019. Highly heterogeneous depleted mantle recorded in the lower oceanic crust. Nature Geoscience. doi:10.1038/s41561-019-0368-9
Lynne J. Elkins, Bernard Bourdon, Sarah Lambart, 2019. Testing pyroxenite versus peridotite sources for marine basalts using U-series isotopes. Lithos. Invited Review. 332-333, 226-244. doi:10.1016/j.lithos.2019.02.011
Sarah Lambart, Heather M. Savage, Ben G. Robinson+, Peter B. Kelemen, 2018. Experimental investigation of the pressure of crystallization of Ca(OH)2: Implications for the reactive-cracking process. Geochemistry, Geophysics, Geosystems 19. doi:10.1029/2018GC007609.
Peter B. Kelemen, R. Aines, E. Bennett, S.M. Benson, E. Carter, J.A. Coggon, J.C. de Obeso, O. Evans, G. Gadikota, G.M. Dipple, M. Godard, M. Harris, J.A. Higgins, K.T.M. Johnson, F. Kourim, R. Lafay, Sarah Lambart, C.E. Manning, J.M. Matter, K. Michibayashi, T. Morishita, J. Noëli, K. Okazaki, P. Renforth, B. Robinson, H. Savage, R. Skarbek, M.W. Spiegelman, E. Takazawa, D. Teagle, J.L. Urai, J. Wilcox and the Oman Drilling Project Phase 1 Scientific Party, 2018. In situ carbon mineralization in ultramafic rocks: Natural processes and possible engineered methods. Energy Procedia 146, 92-102. doi:10.1016/j.egypro.2018.07.013.
Sarah Lambart, 2017. No direct contribution of recycled crust in Icelandic basalts. Geochemical Perspectives Letters 4, 7-12. doi:10.7185/geochemlet.1728.
Sarah Lambart, Michael B. Baker, Edward M. Stolper, 2016. The role of pyroxenite in basalt genesis: Melt-PX, a melting parameterization for mantle pyroxenites between 0.9 and 5 GPa. Journal of Geophysical Research - Solid Earth 121 (8), 5708-5735. doi:10.1002/2015JB012762.
Didier Laporte, Sarah Lambart, Pierre Schiano, Luisa Ottolini, 2014. Experimental derivation of nepheline syenite and phonolite liquids by partial melting of upper mantle peridotites. Earth and Planetary Science Letters 404, 319-331. doi:10.1016/j.epsl.2014.08.002.
Oliver Shorttle, John Maclennan, Sarah Lambart, 2014. Quantifying lithological variability in the mantle. Earth and Planetary Science Letters 395, 24-40. doi:10.1016/j.epsl.2014.03.040.
Sarah Lambart, Didier Laporte, Pierre Schiano, 2013. Markers of the pyroxenite contribution on the major-element compositions of oceanic basalts: Review of the experimental constraints. Lithos 160-161, 14-36. doi:10.1016/j.lithos.2012.11.018.
Sarah Lambart, Didier Laporte, Ariel Provost, Pierre Schiano, 2013. Fate of pyroxenite-derived melts in the peridotitic mantle: Thermodynamic and experimental constraints. Journal of Petrology 53(3), 451-476. doi:10.1093/petrology/egr068.
Sarah Lambart, Didier Laporte, Pierre Schiano, 2009. An experimental study of pyroxenite partial melts at 1 and 1.5 GPa: Implications for the major element composition of Mid-Ocean Ridge Basalts. Earth and Planetary Science Letters 288(1-2), 335-347. doi:10.1016/j.epsl.2009.09.038.
Sarah Lambart, Didier Laporte, Pierre Schiano, 2009. An experimental study of focused magma transport and basalt-peridotite interactions beneath mid-ocean ridges: implications for the generation of primitive MORB compositions. Contributions to Mineralogy and Petrology 157(4), 429-451. doi:10.1007/s00410-008-0344-7.
Reports:
Planke, S., Berndt, C., Alvarez Zarikian, C.A., and the Expedition 396 Scientists, 2023. Mid-Norwegian Margin Magmatism and Paleoclimate Implications. Proceedings of the International Ocean Discovery Program, Volume 396. doi:10.14379/iodp.proc.396.2023
Planke, S., Berndt, C., Alvarez Zarikian, C.A., and the Expedition 396 Scientists, 2022. Expedition 396 Preliminary Report: Mid-Norwegian Continental Margin Magmatism and Paleoclimate Implications. International Ocean Discovery Program. doi:10.14379/iodp.pr.396.2022
Conference Abstracts:
* Speaker, + Student
Otto Lang+ & Sarah Lambart*, 2021. Partitioning of first row transition elements in mantle lithologies.https://doi.org/10.7185/gold2021.7709
Juliette Pin*, Lyderic France, Laurie Reisberg & Sarah Lambart, 2021. Thermodynamic model for the refertilization of the non-cratonic continental mantle lithosphere.https://doi.org/10.7185/gold2021.5861
Otto Lang*+ & Sarah Lambart, 2020. Identifying lithological tracers with first row transition element partitioning of natural pyroxenites. AGU FM 2020, Abstract# V038-014.
Sarah Lambart* & Otto Lang+, 2020. Lithological heterogeneities in the mantle: origins and contributions to magma genesis. Goldschmidt 2020, virtual, Jun. 2020, doi: 10.46427/gold2020.1405. Keynote talk
Sarah Hamilton+ & Sarah Lambart*, 2019. Compositional variability of San Carlos olivine.
Theses:
Media coverage and Highligths:
Aug 21, 2023 - @THEU: Ancient volcanism drove ancient global warming that marked the end of the Paleocene
Jan 20, 2022 - @THEU: U geoscientist sails on Arctic research cruise
Jul 19, 2021 - @THEU: U professor to sail on expedition sampling the rocks of the seafloor
Jul 19, 2021 - @THEU: How 3-D modeling helped U geologists teach during COVID-19
May 28, 2019 - @THEU: A chemical mosaic under our feet
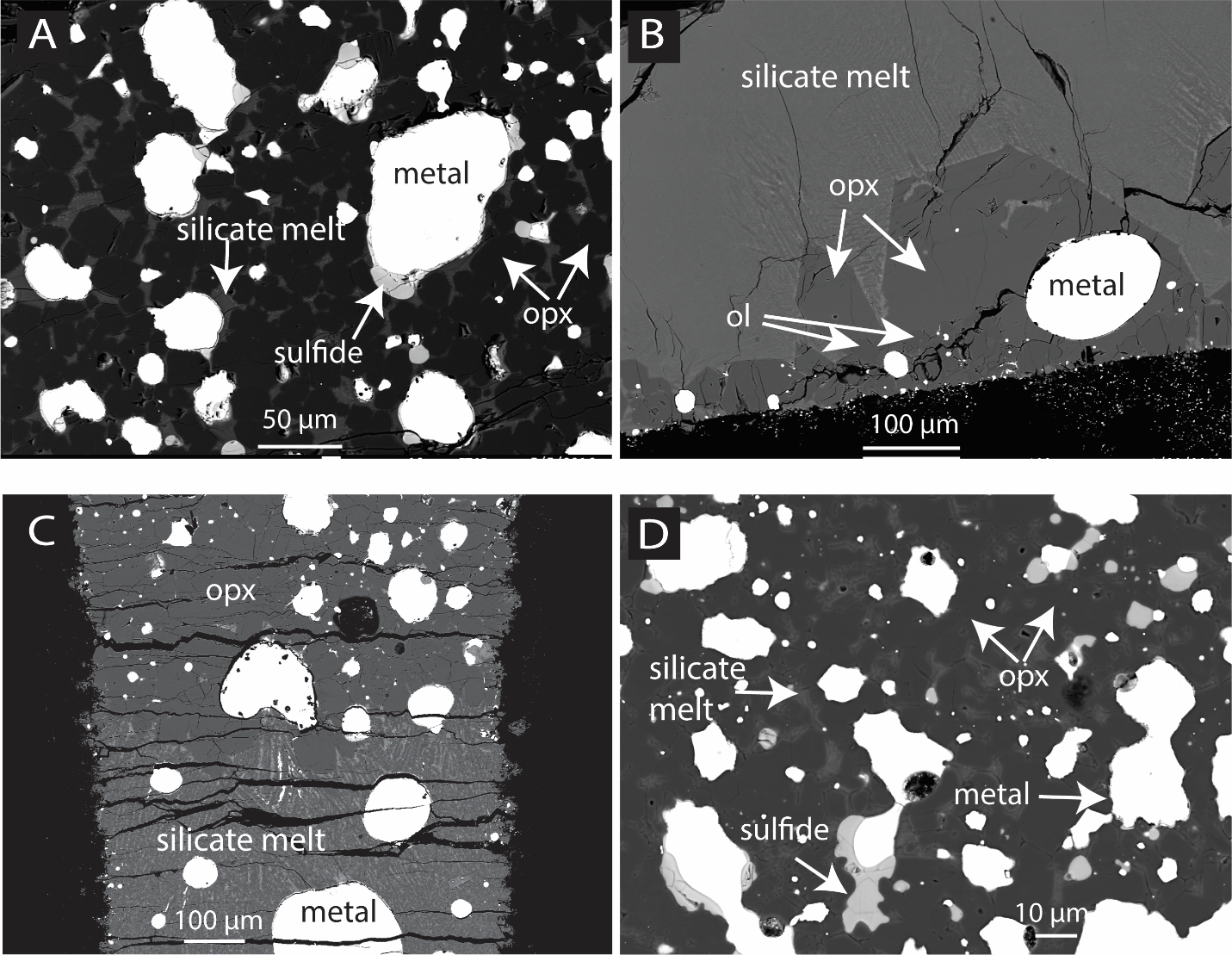
Icarus Abstract: Back-scattered electron images showing the textures of experimental charges.
Volumme 437, 116602, 2025
A Pyroxenite Mantle on Mercury? Experimental Insights from Enstatite Chondrite Melting at Pressures up to 5 GPa.
Asmaa Boujibar, Kevin Righter, Emmanuel Fontaine, Max Collinet, Sarah Lambart, Larry R. Nittler, Kellye M. Pando, Lisa R. Dianelson
Enstatite chondrites are potential source material for the accretion of Mercury due to their reduced nature and enrichment in volatile elements. Understanding their melting properties is therefore important to better assess a scenario where Mercury formed from these chondrites. Here, we present experimental data on the partial melting of a modified EH4 Indarch enstatite chondrite, which was adjusted to have 18 times more metallic Si than SiO2 in mass. Experiments were performed from 0.5 to 5 GPa using piston cylinder and multi-anvil apparatuses. Results indicate that the stability field of enstatite expands relative to olivine at pressures of 0.5 to 1 GPa. This expansion is likely due to the presence of Ca-S and Mg-S complexes in the silicate melt, which enhance SiO2 activity and promote enstatite crystallization. Silicate melts present a correlation between Ca and S concentrations, mirroring the global patterns seen on Mercury's surface. Additionally, sulfides show enrichment in Mg and Ca, up to 22 and 13 wt% respectively. These high Mg and Ca contents are observed at low temperatures and high silica content in the silicate melt, respectively. Partial melting of this reduced EH4 chondrite yields a large range of silicate melt compositions, due to the Mg- and Ca-rich sulfides which act as significant residual phases. High-pressure melts (2 to 5 GPa, 160-400 km depth in Mercury) are Mg-rich, similar to those in Mercury’s high-magnesium region (HMR), while low-pressure melts (0.5 to 1 GPa, 40-80 km depth) are Si-rich, comparable to the northern volcanic plains (NVP). Results suggest that a large fraction of Mercury's surface aligns compositionally with these melts, implying that Mercury’s mantle could predominantly have a pyroxenitic composition. However, regions with differing compositions, such as aluminum-rich areas, like the Caloris basin and areas close to the South pole, suggest local variability in mantle geochemistry. The HMR chemistry indicates melting at pressures up to the base of Mercury’s mantle, possibly due to a large impact. Our study also explores whether the surface compositions could result from mixing processes like impact gardening or polybaric melting and magma mixing. The findings suggest that areas such as the intercrater plains and heavily cratered regions could be mixtures of melts from different depths, ranging from 0.5 to 5 GPa, which corresponds to the core-mantle boundary to lower pressures. Overall, our results support the idea that much of Mercury’s mantle retained a pyroxenite composition, likely due to the extensive stability of enstatite. If mantle stratification occurred during the crystallization of a magma ocean, it may have been controlled by sulfide rather than traditional silicate fractionation, with stratified layers more likely forming near the crust-mantle boundary, where sulfides are more likely to form.
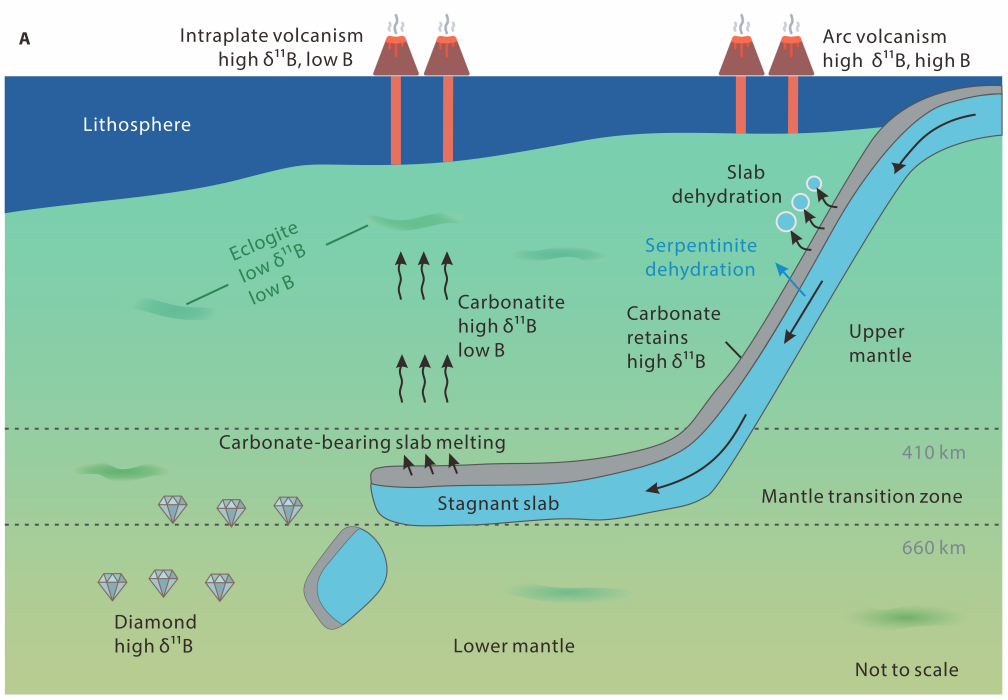
Science Advances Abstract: Conceptual cartoon and geophysical tomography describing the generation of high d11B intraplate magmas.
Volumme 11,eads5104, 2025
Heavy boron isotopes in intraplate basalts reveal recycled carbonate in the mantle.
Rong Xu, Yue Cai, Sarah Lambart, Chunfei Chen, Jun-Bo Zhang, Mei-Fu Zhou, Jia Liu, Zhongjie Bai, Tao Wu, Feng Huang, Ting Ruan, Yongsheng Liu
Recycling of surficial volatiles such as carbon into the mantle plays a fundamental role in modulating Earth’s habitability. However, slab devolatilization during subduction could prevent carbon from entering the deep mantle. Boron isotopes are excellent tracers of recycled volatiles, but correlations between boron isotopes and mantle heterogeneity indicators are rarely observed, thereby casting doubt that substantial amounts of volatiles and boron can be recycled into the deep mantle. Here we show that boron isotopes in two different types of primitive continental intraplate basalts correlate well with mantle heterogeneity indicators, indicating contributions of various subducted crustal components. Most importantly, a common high d11B component shared by both types of basalts is best explained as recycled subducted carbonate rather than serpentinite. Our findings demonstrate that subducted carbonate carries heavy B into Earth’s deep mantle, and its recycling could account for the high d11B signatures observed in intraplate magmas and deeply-sourced carbonatites.
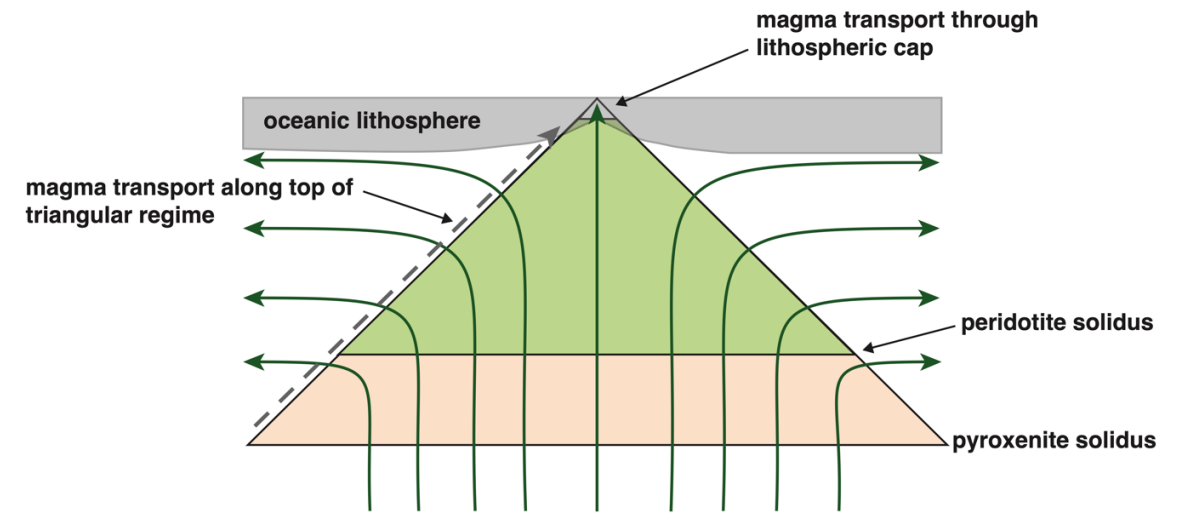
Volcanica Abstract: Cartoon illustrating a bilithologic triangular melting regime beneath mid-ocean ridges and showing paths along which extracted melts may experience radioactive decay during transport.
Volumme 7(2), 685-715, 2024
Uranium-series disequilibria in MORB, revisited: A systematic numerical approach to partial melting of a heterogeneous mantle.
Lynne J. Elkins, Sarah Lambart
Here we present systematic, computational modeling outcomes for bilithologic mantle melting in divergent midocean ridge environments. We present outcomes for equilibrium and disequilibrium porous flow melting for mantle containing 0-50% pyroxenite in thermal equilibrium with fertile peridotite, for potential temperatures of 1300 and 1400 °C, solid upwelling rates from 1-50 cm/yr., and residual maximum porosities of 0.1-2.0%. Our calculations support a multi-lithologic, globally heterogeneous mantle that also melts in a heterogeneous manner. Melting of peridotite alone can reproduce some but not all global MORB data. Silica-excess pyroxenites can uniquely produce low (226Ra/230Th) and (231Pa/235U) with high (230Th/238U), but quantities greater than ~10% also produce anomalously thick crust, restricting their likely abundance. Silica-deficient pyroxenites produce more moderate outcomes, except for chemical disequilibrium transport scenarios with unrealistically high (231Pa/235U). We also show that U-series disequilibria in partial melts can be decoupled from trace element compositions by radioactive decay during transport in two-dimensional regimes.
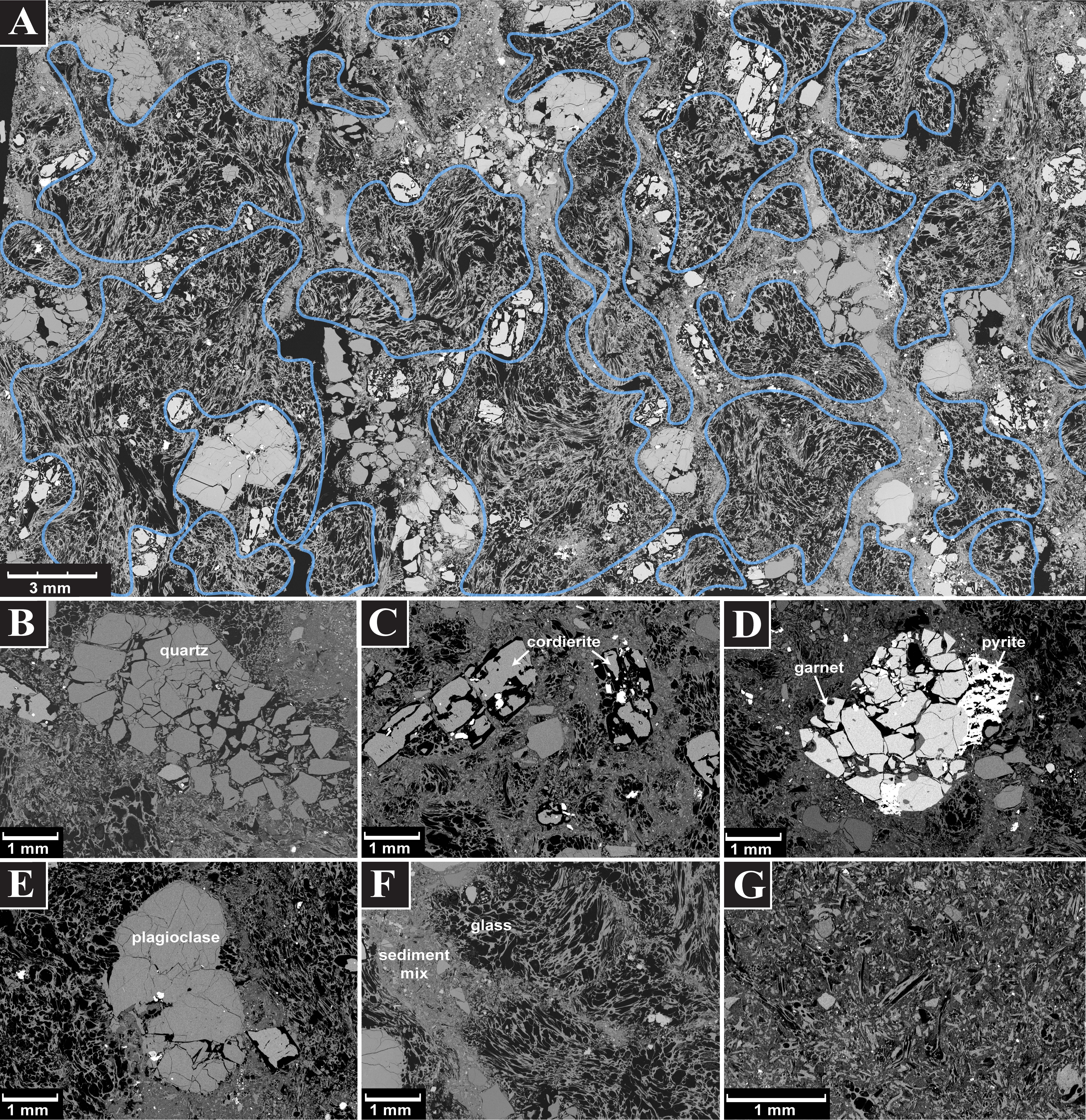
Geochemistry, Geophysics, Geosystems Abstract: Plain Language Summary: BSE images highlighting various textures observed in the dacitic unit.
Volumme 25 (7), e2023GC011413, 2024
Evidence for Low-pressure Crustal Anatexis During the Northeast Atlantic Break-up.
Ashley M. Morris, Sarah Lambart, Michael A. Stearns, John R. Bowman, Morgan T. Jones, Geoffroy T.F. Mohn, Graham Andrews, John Millet, Christian Tegner, Sayantani Chatterjee, Joost Frieling, Pengyuan Guo, David W. Jolley, Emily H. Cunningham, Christian Berndt, Sverre Planke, Carlos A. Alvarez Zarikian, Peter Betlem, Henk Brinkhuis, Marialena Christopoulou, Eric Ferré, Irina Y. Filina, Dustin Harper, Jack Longman, Reed P. Scherer, Natalia Varela, Weimu Xu, Stacy L. Yager, Amar Agarwal, Vincent J. Clementi
While basaltic volcanism is dominant during rifting and continental breakup, felsic magmatism may be a significant component of some rift margins. During International Ocean Discovery Program (IODP) Expedition 396 on the continental margin of Norway, a graphite-garnet-cordierite bearing dacitic unit was recovered within Early Eocene sediments on Mimir High (Site U1570), a marginal high on the Vøring Transform Margin. Here, we present a comprehensive textural, petrological, and geochemical study of the dacite in order to assess its origin and discuss the geodynamic implications. The major mineral phases (garnet, cordierite, quartz, plagioclase, alkali feldspar) are hosted in a fresh rhyolitic, vesicular, glassy matrix that is locally mingled with sediments. The major element chemistry of garnet and cordierite, the presence of zircon inclusions with inherited cores, and thermobarometric calculations all support an upper crustal metapelitic origin. While most magma-rich margin models favor crustal anatexis in the lower crust, thermobarometric calculations performed here show that the dacite was produced at upper-crustal depths ( < 5 kbar, 18 km depth) and high temperature (750-800 °C) with up to 3 wt% water content. In situ U-Pb analyses on zircon inclusions give a magmatic age of 54.6 ± 1.1 Ma,revealing the emplacement of the dacite post-dates the Paleocene-Eocene Thermal Maximum (PETM). Our results suggest that the opening of the Northeast Atlantic was associated with a phase of low-pressure, high-temperature crustal anatexis at the onset of the main phase of magmatism
Fifty-six million years ago, the continents were beginning the final phase of their journey to their modern-day locations. This included the rifting and formation of the Northeast Atlantic Ocean, known in particular for producing excess amounts of magma during continental break-up. The International Ocean Discovery Program organized Expedition 396 to collect volcanic and sedimentary rocks deposited during this time off the coast of present-day Norway to investigate the cause of the excess magmatism and its potential implications for the global climate. While sampling sediments on the expedition, an unexpected volcanic unit, a glassy garnet-cordierite dacite, was recovered. To determine its origin, we combined multiple methods (petrography, stratigraphy, thermodynamic calculations, geochronology, in situ compositional analyses) and showed that the unit is a product of melting of in the continental crust during the rifting process and likely later emplaced in shallow water. Our results demonstrate that the rifting process in the Northeast Atlantic included a long and intense period of continental crustal thinning. This research provides evidence needed to reconstruct the evolution of the North Atlantic Ocean.
Gondwana Research Abstract: Graphical abstract.
Volumme 135, 180-197, 2024
From an accretionary margin to a sediment-rich collision: Spatiotemporal evolution of the magmatism during the closure of the Mongol-Okhotsk Ocean.
Henriquez S., Ochir G., Lambart S., Johnson C.L., Webb L.E., Lippert P.C.
The closure of the Mongol-Okhotsk Ocean (MOO) marks the final suturing of the Central Asian Orogenic Belt, one of the largest accretionary orogens on Earth and a region that is considered an archetype for crustal growth during the Phanerozoic. Abundant Permian to Triassic magmatism in Mongolia extended into the Jurassic on the eastern side of the Mongol-Okhotsk Belt (MOB), the orogenic belt produced by the closure of the MOO. Magmatic belts formed north and south of the suture along the MOB provide insight into the dynamics of the subduction system and the magmatic, crustal, and mantle processes pre-, syn- and post- collision within this accretionary margin. One of the main questions regarding the magmatism in the region is: Was the magmatism formed during active subduction or during the collision and closure of the basin? Here we compile geochemical data (major and trace elements, and isotopes) from the Permian to Jurassic magmatic rocks in the MOB and analyze their spatiotemporal characteristics. Our goal is to assess how magmatism changed in time and space during the closure of the Mongol-Okhotsk Ocean and how those changes relate to first-order tectono-magmatic processes right before or during the collisional event that closed the basin. Our results show a general enrichment in fluid mobile elements, LILE, and LREE and depletion in HFSE, and HREE in mafic and felsic rocks, which indicates a mantle metasomatized by subduction-related fluids regardless of crustal contamination. Our analysis supports higher enrichment in sediment melts, especially along its western and older extent, and the assimilation of juvenile crustal components without producing abundant S-type peraluminous magmatism which indicates mantle and crustal contributions. Thus, we conclude that magmatism formed above a sediment-rich retreating margin was able to recycle and stabilize young and compositionally evolved crustal material in the Central Asian Orogenic Belt.

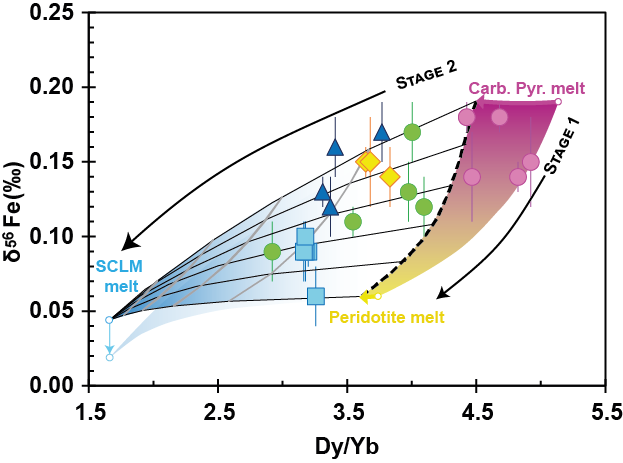
Earth and Planetary Science Letters Abstract: Variation of δ56e vs. Dy/Yb for the studied continental intraplate basalts compared with the 2-stage model described in the manuscript.
Volumme 624, 118499, 2024
Iron isotope evidence on continental intraplate basalts for mantle lithosphere imprint on heterogenous asthenospheric melts.
Xu R., Lambart S., Nebel O., Li M., Bai Z., Zhang J., Zhang G., Goa J., Zhong H., Liu Y.
Iron isotope studies on ocean island basalts (OIBs) and mid-ocean ridge basalts (MORBs) have disclosed the contribution of pyroxenite lithologies in the generation of basaltic magmas. Whether Fe isotopic compositions of continental intraplate basalts can be applied to trace lithological heterogeneity within the mantle source regions remains unclear. To explore the systematics of stable Fe isotopes as a potential probe of lithological heterogeneity in the source of continental intraplate basalts, we present twenty-four Fe isotope data on a suite of well-characterized Cenozoic basalts from SE China. The samples show a large range of Fe isotope values (δ56Fe = +0.09‰ to +0.20‰), which correlate significantly with SiO2, CaO/Al2O3, Ti/Eu, Hf/Hf*, Zr/Nb, Dy/Yb, La/Yb, Nb/Y, K/La, Sr/Ce, δ66Zn and estimated equilibrium pressures. The samples with the heaviest δ56Fe values represent early-stage low-silica basalts with moderately enriched Sr-Nd isotope ratios and high δ66Zn values, while the late-stage high-silica basalts display a broad δ56Fe decrease with increasing 87Sr/86Sr and with decreasing εNd and δ66Zn. We demonstrate that the heaviest Fe isotope signatures cannot be derived from a pure peridotite source, independently of the degree of oxidation and of the pressure of melting, but instead are consistent with low-degree melt produced by the adiabatic decompression of a carbonated pyroxenite-bearing asthenospheric mantle. Mixing of these hybrid melts with melt produced from in situ melting of the sub-continental lithospheric mantle (SCLM) reproduces the Fe-isotope variability of the late-stage samples. Our results demonstrate the significance of lithological heterogeneity in the mantle source of continental basalts and highlights the subsequent imprint of mantle lithosphere in the evolution of their Fe isotope compositions. Finally, this study exemplifies the potential of the Fe-Zn stable isotope pair for mantle geochemistry; coupled with more traditional radiogenic isotopes and major and trace element concentrations, they formed an efficient tool to trace the nature and the contribution of the mantle source components in the formation of intraplate basalts.
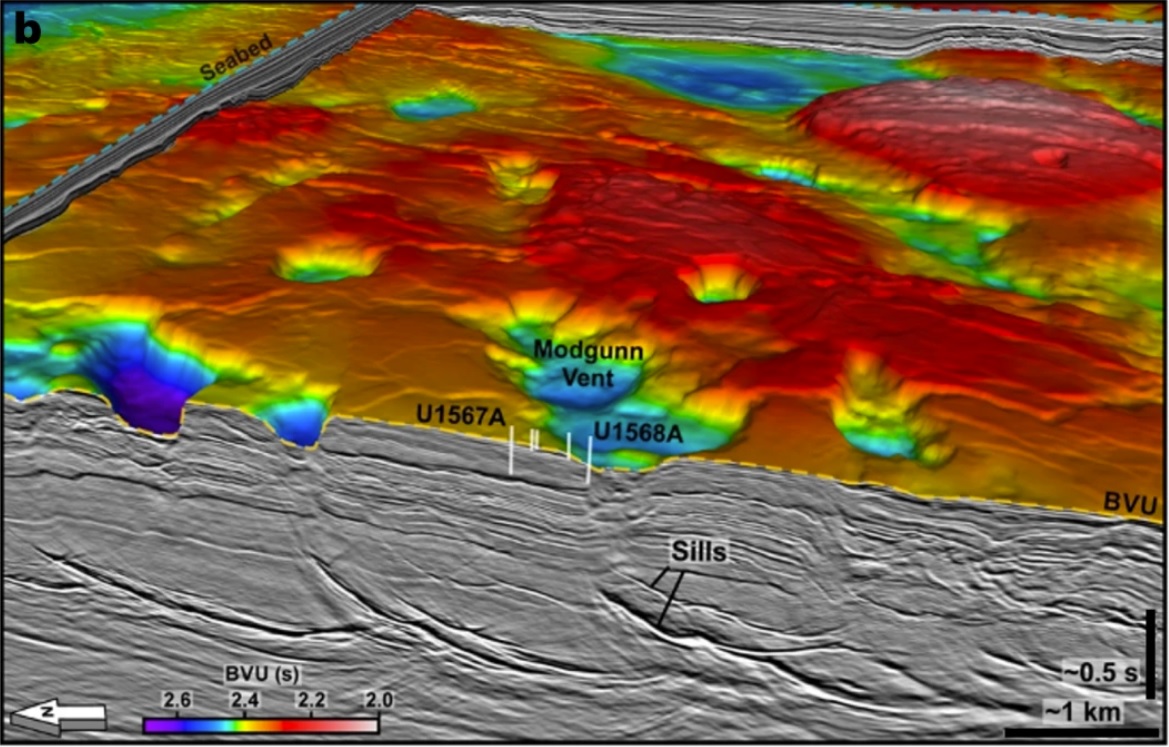
Nature Geoscience Abstract: Three-dimensional view of seismic reflection data showing the relationship between sills and the hydrothermal vents.
Volumme XXX, XXX,3 August 2023
Shallow-water hydrothermal venting linked to the Palaeocene–Eocene Thermal Maximum.
Christian Berndt, Sverre Planke, Carlos A. Alvarez Zarikian, Joost Frieling, Morgan T. Jones, John M. Millett, Henk Brinkhuis, Stefan Bünz, Henrik H. Svensen, Jack Longman, Reed P. Scherer, Jens Karstens, Ben Manton, Mei Nelissen, Brandon Reed, Jan Inge Faleide, Ritske S. Huismans, Amar Agarwal, Graham D. M. Andrews, Peter Betlem, Joyeeta Bhattacharya, Sayantani Chatterjee, Marialena Christopoulou, Vincent J. Clementi, Eric C. Ferré, Irina Y. Filina, Pengyuan Guo, Dustin T. Harper, Sarah Lambart, Geoffroy Mohn, Reina Nakaoka, Christian Tegner, Natalia Varela, Mengyuan Wang, Weimu Xu & Stacy L. Yager
The Palaeocene–Eocene Thermal Maximum (PETM) was a global warming event of 5–6°C around 56 million years ago caused by input of carbon into the ocean and atmosphere. Hydrothermal venting of greenhouse gases produced in contact aureoles surrounding magmatic intrusions in the North Atlantic Igneous Province have been proposed to play a key role in the PETM carbon-cycle perturbation, but the precise timing, magnitude and climatic impact of such venting remains uncertain. Here we present seismic data and the results of a five-borehole transect sampling the crater of a hydrothermal vent complex in the Northeast Atlantic. Stable carbon isotope stratigraphy and dinoflagellate cyst biostratigraphy reveal a negative carbon isotope excursion coincident with the appearance of the index taxon Apectodinium augustum in the vent crater, firmly tying the infill to the PETM. The shape of the crater and stratified sediments suggests large-scale explosive gas release during the initial phase of vent formation followed by rapid, but largely undisturbed, diatomite-rich infill. Moreover, we show that these vents erupted in very shallow water across the North Atlantic Igneous Province, such that volatile emissions would have entered the atmosphere almost directly without oxidation to CO2 and at the onset of the PETM.
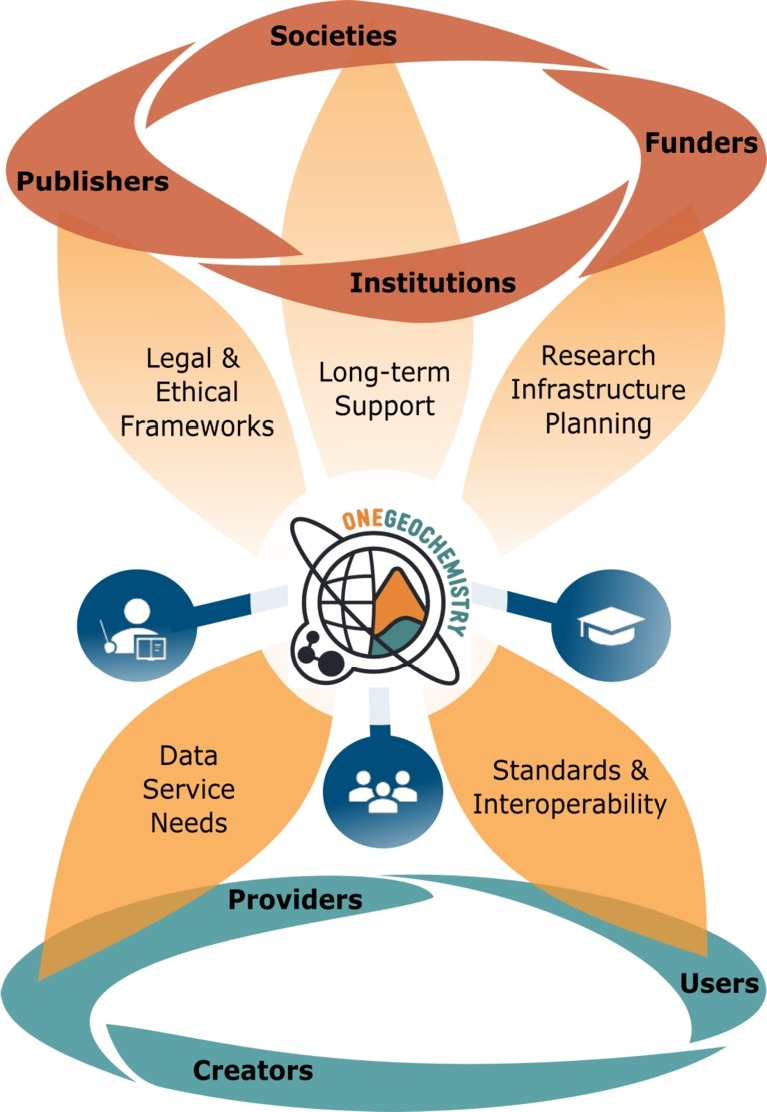
Geochimica et Cosmochimica Acta Abstract: The place of OneGeochemistry within the broader research data landscape.
Volumme 351, 192-205, 28 April 2023
Community recommendations for geochemical data, services and analytical capabilities in the 21st century.
Marthe Klöcking, Lesley Wyborn, Kerstin A. Lehnert, Bryant Ware, Alexander M. Prent, Lucia Profeta, Fabian Kohlmann, Wayne Noble, Ian Bruno, Sarah Lambart, Halimulati Ananuer, Nicholas D. Barber, Harry Becker, Maurice Brodbeck, Hang Deng, Kai Deng, Kirsten Elger, ..., Tengfei Zhou
The majority of geochemical and cosmochemical research is based upon observations and, in particular, upon the acquisition, processing and interpretation of analytical data from physical samples. The exponential increase in volumes and rates of data acquisition over the last century, combined with advances in instruments, analytical methods and an increasing variety of data types analysed, has necessitated the development of new ways of data curation, access and sharing. Together with novel data processing methods, these changes have enabled new scientific insights and are driving innovation in Earth and Planetary Science research. Yet, as approaches to data-intensive research develop and evolve, new challenges emerge. As large and often global data compilations increasingly form the basis for new research studies, institutional and methodological differences in data reporting are proving to be significant hurdles in synthesising data from multiple sources. Consistent data formats and data acquisition descriptions are becoming crucial to enable quality assessment, reusability and integration of results fostering confidence in available data for reuse. Here, we explore the key challenges faced by the geo- and cosmochemistry community and, by drawing comparisons from other communities, recommend possible approaches to overcome them. The first challenge is bringing together the numerous sub-disciplines within our community under a common internationally initiative. One key factor for this convergence will be gaining endorsement from the international geochemical, cosmochemical and analytical societies and associations, journals and institutions. Increased education and outreach, spearheaded by ambassadors recruited from leading scientists across disciplines, will further contribute to raising awareness, and to uniting and mobilising the community. Appropriate incentives, recognition and credit for good data management as well as an improved, user-oriented technical infrastructure will be essential for achieving a cultural change towards an environment in which the effective use and real-time interchange of large datasets is common-place. Finally, the development of best practices for standardised data reporting and exchange, driven by expert committees, will be a crucial step towards making geo- and cosmochemical data more Findable, Accessible, Interoperable and Reusable by both humans and machines (FAIR).
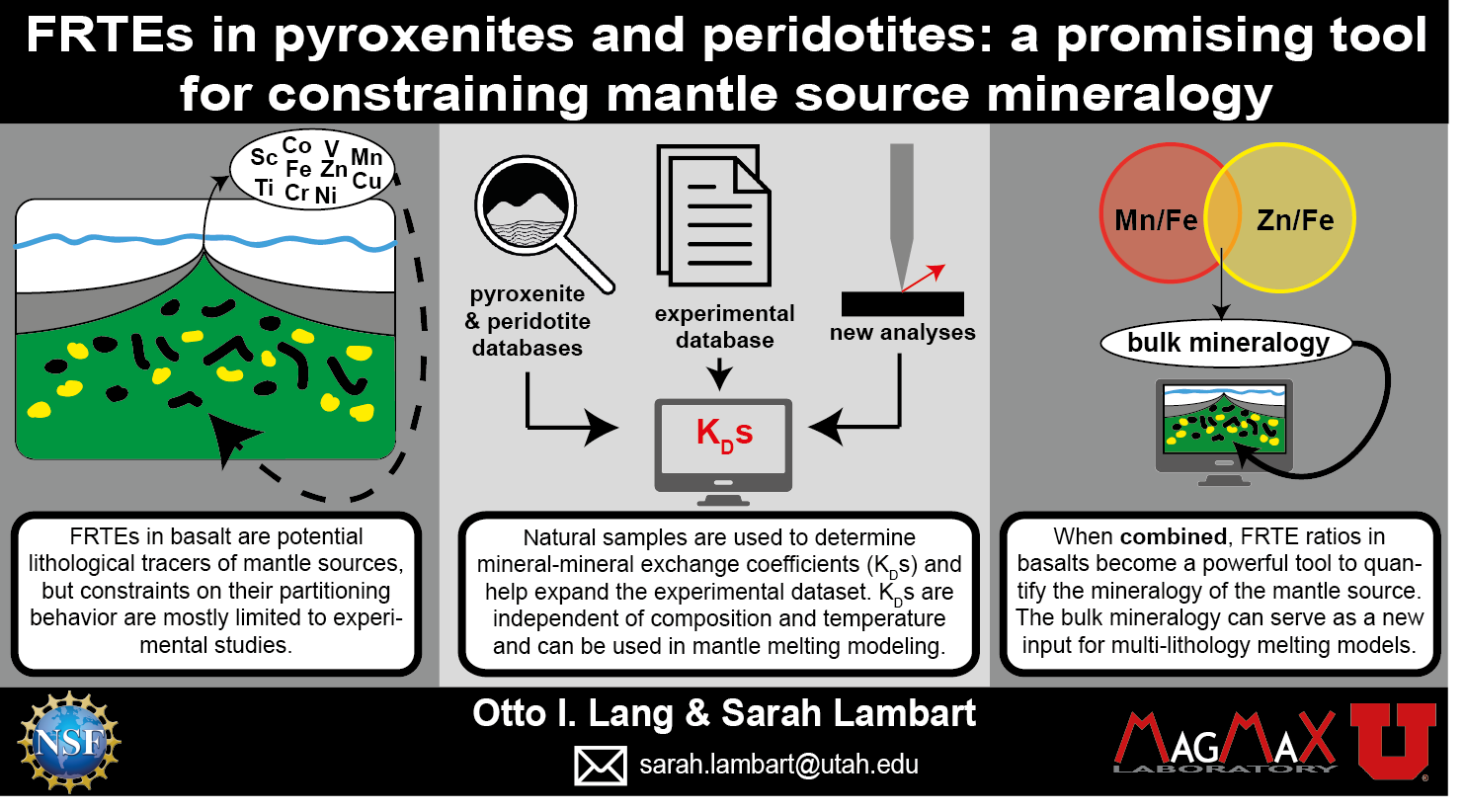
Chemical Geology Invited Research Article Abstract: Graphical Abstract.
Volumme XXX, 121137,XX October 2022
First-row transition elements in pyroxenites and peridotites: A promising tool for constraining mantle source mineralogy.
Otto I. Lang, Sarah Lambart
Mantle heterogeneity has a first-order control on the petrological and geochemical differences of erupted mafic lavas worldwide. Because of their contrasted distributions between mantle phases, First Row Transition Elements (FRTEs) have been considered potential lithological tracers. Using a combination of published data on natural and experimental samples and new high-precision analyses on a variety of pyroxenite samples, we investigated the parameters that control FRTE partition (Ds) and exchange (KDs) coefficients between common mantle minerals. We demonstrate that mineral-clinopyroxene exchange coefficients are independent of composition and temperature and that coefficients obtained from natural samples can be accurate as long a sufficiently high number of compositionally diverse samples are considered, making them reliable input parameters in mantle melting models. As a proof of concept, we used the exchange coefficients determined from natural mantle lithologies in this study, along with published experimental clinopyroxene/melt partitioning coefficients, to perform simple inverse modeling on two basalt suites from the Western Volcanic Zone in Iceland and Samoa, selected for their contrasted Mn/Fe and Zn/Fe ratios. Our results show that a given FRTE ratio in basalt can be explained by a large range of modal proportions in the source. However, when combined, FRTE ratios become a powerful tool to constrain the nature of the source.
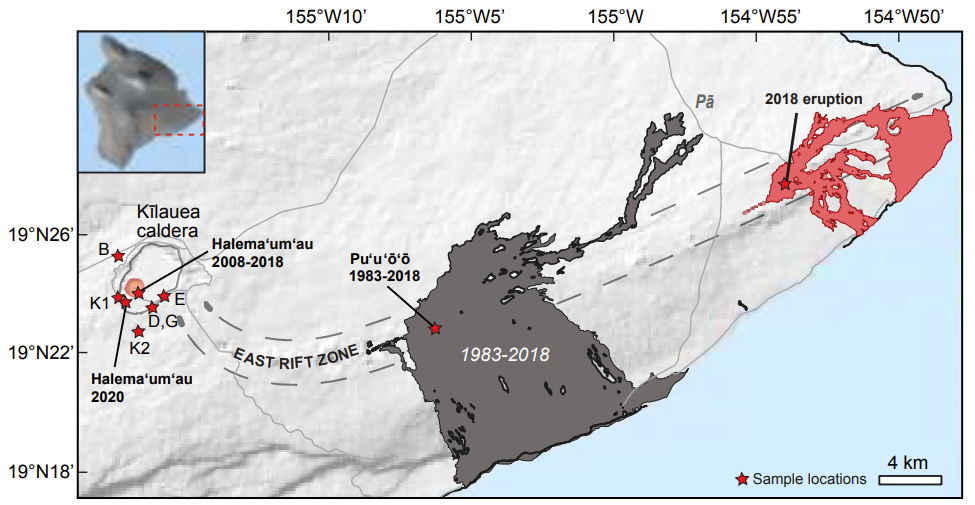
Earth and Planetary Science Letters Abstract: Map of Kılauea Volcano with the studied eruptions.
Volumme 595, 117769,1 October 2022
Trace elements in olivine fingerprint the source of 2018 magmas and shed light on explosive-effusive eruption cycles at Kılauea Volcano.
Adrien J. Mourey, Thomas Shea, Kendra J. Lynn, Allan H. Lerner, Sarah Lambart, Fidel Costa, Jeffrey Oalmann, R. Lopaka Lee, Cheryl Gansecki
Understanding magma genesis and the evolution of intensive parameters (temperature, pressure, composition, degree of melting) in the mantle source of highly active volcanic systems is crucial for interpreting magma supply changes over time and recognizing cyclic behavior to anticipate future volcanic behavior. Major and trace elements in olivine are commonly used to study variations in mantle lithologies and melting conditions (e.g., temperature, pressure, oxygen fugacity) affecting the mantle over time. Here, we track the temporal evolution of primary melts through the most recent cycle of explosive and effusive eruptions at Kılauea (Hawaii), which spans the last ∼500 years. We report major and trace elements in olivine from the last explosive period (1500-early 1820’s Keanakakoi Tephra) and the most recent decade of the current effusive period (2018 LERZ, 2015-2018 Pu‘u‘o‘o, 2008-2018 lava lake and 2020 eruption in Halema‘uma‘u). Scandium concentra28 tions in olivine allow characterizing changes in mantle source between 1500 and 2018, and suggest that the recent (2015-2018) magma feeding the Pu‘u‘o‘o cone did not significantly interact with the magma that erupted in the LERZ in 2018. The evolution of olivine and melt compositions over the past 500 years is not easily reconcilable with variations in mantle potential temperature, pressure of mantle melt pooling and storage, or even oxygen fugacity conditions. Instead, Sc, Mn, and Co concentrations and Ni/Mg ratio in high forsterite (Fo>87) olivine advocate for an increase in the proportion of clinopyroxene in the mantle source associated with a slightly higher degree of partial melting from 1500 to 2018. Changes in primitive melt compositions and degrees of mantle melting may well modulate magma supply to the crust and formation-replenishment of steady or ephemeral summit reservoirs, and thereby control transitions between explosive and effusive periods at K¯ılauea. Analyzing trace elements in olivine at Kılauea and elsewhere could therefore provide important clues on subtle changes occurring at the mantle level that might herald changes in volcanic behavior.
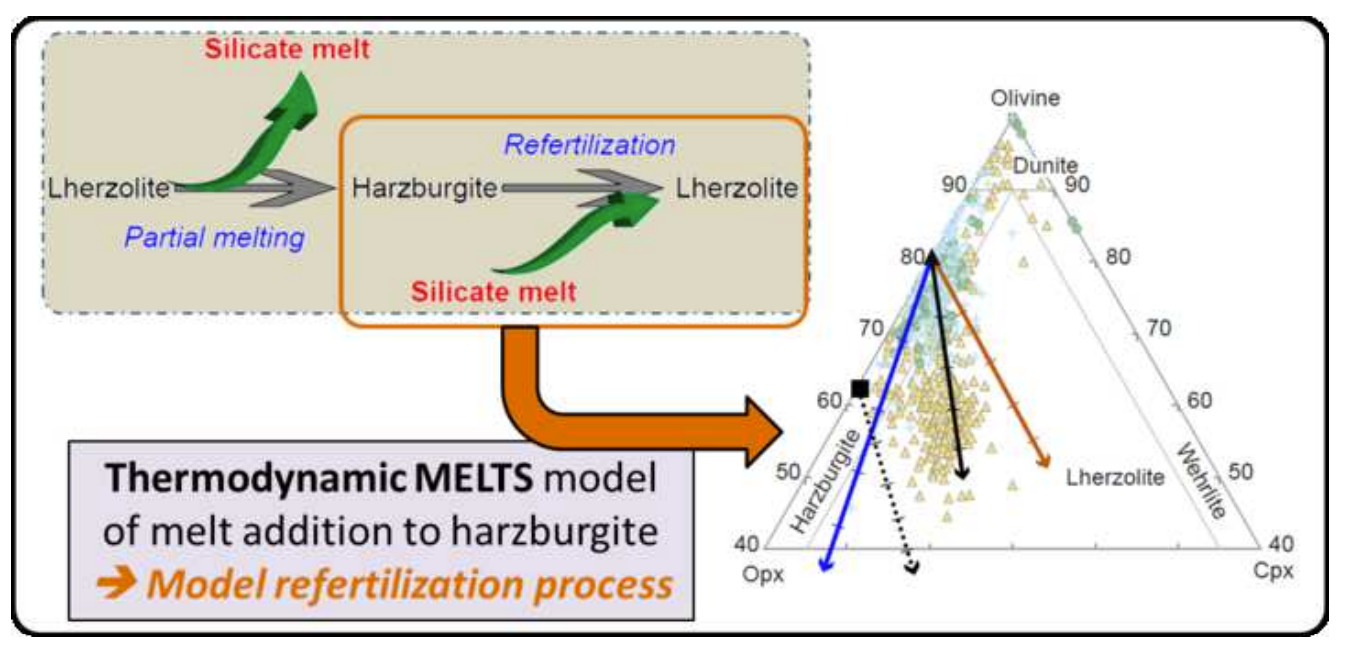
Chemical Geology Abstract: Graphical abstract.
Volumme 609, 121050,20 October 2022
Thermodynamic modeling of melt addition to peridotite: implications for the refertilization of the non-cratonic continental mantle lithosphere.
Juliette Pin, Lyderic France, Sarah Lambart, Laurie Reisberg
In a classic model of evolution of the non-cratonic continental mantle lithosphere, harzburgites represent the refractory (< 5% clinopyroxene) residues of high degrees of partial melting of fertile mantle, while lherzolites (> 5% clinopyroxene) represent residues of lesser degrees of partial melting. However, partial melting is not the only process that could explain the peridotite compositional variability that ranges from fertile (> 2 wt.% Al2O3, <45 wt.% MgO) to refractory (< 2 wt.% Al2O3, > 45 wt.% MgO). In the refertilization process, harzburgite is a refractory protolith (potentially previously formed by partial melting of a fertile mantle) that undergoes reactive percolation of silicate melts derived from the underlying asthenosphere, resulting in the crystallization of a new generation of minerals (mostly clinopyroxene). A simple but critical first step towards understanding the refertilization process is to examine how modal and major element compositions evolve as melts are added to peridotites. Here we use a thermodynamically-constrained two-component mixing model to independently evaluate the roles of five different parameters: pressure, temperature, redox conditions, and compositions of the initial peridotite and the added basaltic melt (hereafter referred to P-T-fO2-Xπ-Xmelt), during melt addition. We compare the results with observed suites of peridotites. The main observations are as follows: (1) the produced model is consistent with the global peridotite database, and (2) T, fO2 and small variations of pressure have almost no impact on the evolution of the system. In contrast, the mineralogy of the percolated harzburgite has a substantial effect on the variation of the modal proportions. The parameter with the most significant impact is Xmelt, which is directly linked to the geodynamic context and melting conditions. This parameter directly controls the refertilization reaction and so, the phase proportions and the bulk-rock composition. Elements that partition preferentially in the melt phase (e.g., Na) display depletions in natural assemblages that are stronger than those predicted from the simple mixing model, consistent with the fact that the natural process occurs in an open system, and that reactive percolation likely results in incompatible element enrichment in the associated melt. Our results corroborate the suggestion that most of the spectrum of compositional variability observed in lithospheric mantle peridotites can be explained by the impregnation of primitive silicate melt in refractory harzburgites.
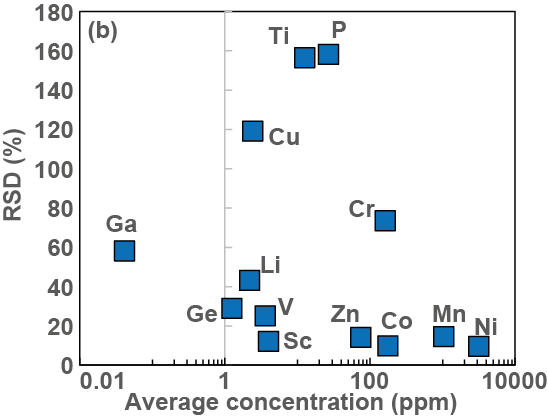
Chemical Geology Abstract: Relative variability of the concentrations between individual Fo-rich grains of San Carlos olivine as a function of the average concentration in the same grains for each element.
Volumme 605, pages 120968,6 June 2022
Compositional variability of San Carlos olivine.
Sarah Lambart, Sarah Hamilton, Otto I. Lang
Forsterite (Fo)-rich olivine compositions from San Carlos (Arizona, SW USA) are commonly used as starting material in experimental petrology. In comparison to the San Carlos reference material USNM 111312/444, it has been shown that the major element variability of non-USNM San Carlos olivine is significant. We complement the characterization of the compositional variability of the non-USNM San Carlos olivine with new data, including minor and trace element analyses. High precision analyses reveal that selected minor elements (e.g., ∼6% NiO, ∼10% MnO, ∼16% CaO, relative) and trace elements (e.g., ∼75% Cr, ∼120% Cu, ∼160% P and Ti, relative) present significant concentration variations between grains. At the scale of the individual grain, however, San Carlos Fo-rich olivines appear relatively homogeneous with no systematic core-rim variations. We also discuss the origin of olivine pyroxenites associated with the peridotite xenoliths and argue that they are derived by melt-rock reaction resulting in olivine dissolution and pyroxene precipitation from the peridotite host. Further segregation and in situ crystallization of the hybrid residual melt can produce Fe-rich olivine-poor pyroxenites. Finally, we discuss the origin of the P variability in San Carlos mantle olivine and suggest that P enrichment by metasomatism implies a highly reactive process with fast dissolution-reprecipitation of solid phases.
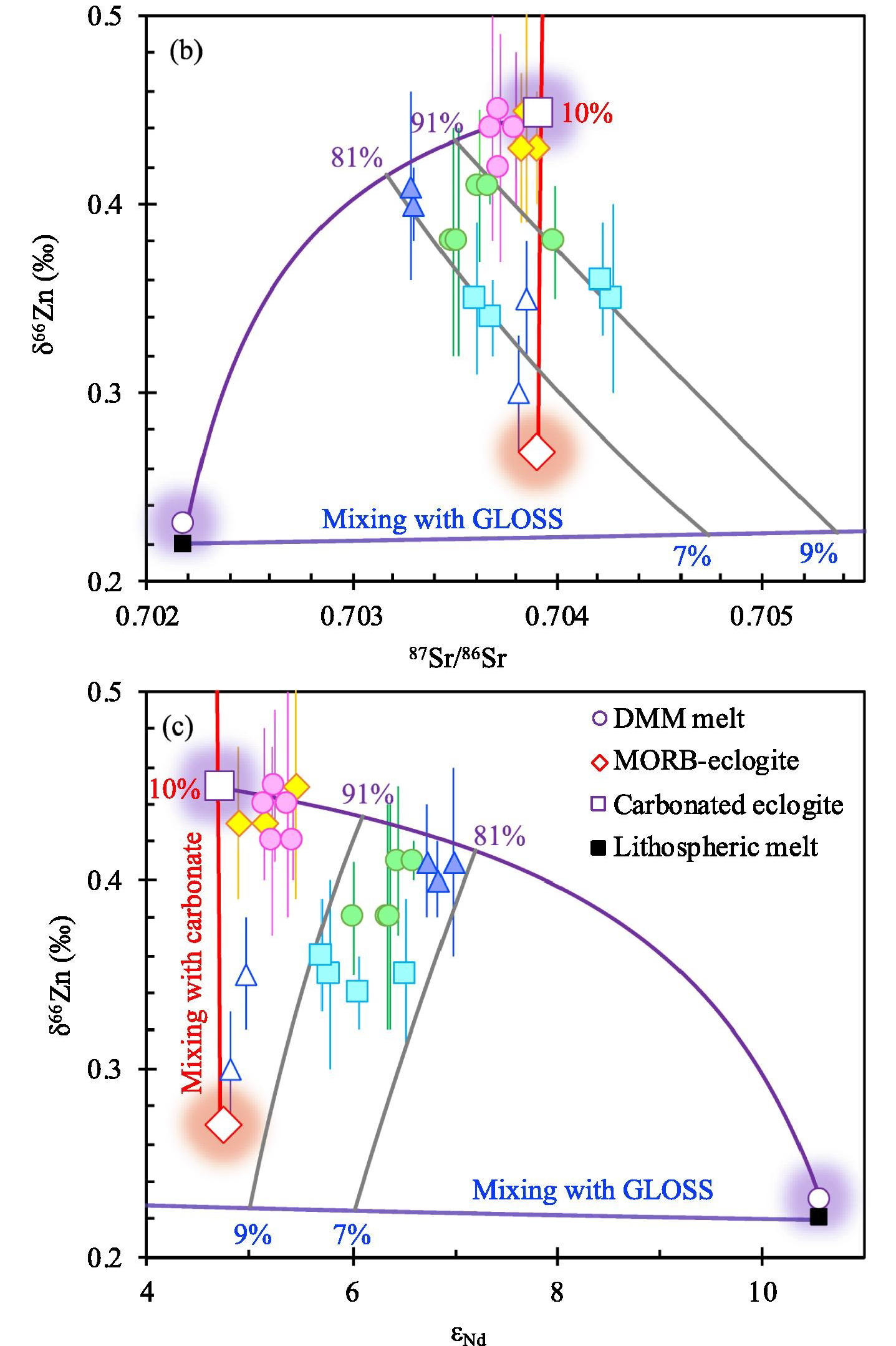
Geochimica et Cosmochimica Acta Abstract: Variation of δ66Zn as a function of 87Sr/86Sr and εNd for the studied basalts. The red line illustrates mixing between MORB-like eclogite and carbonate components to form the carbonated eclogite component. The purple curve represents the mixing line between DMM and carbonated eclogite components. The blue line represents mixing between the lithospheric mantle and GLOSS components and the gray lines illustrate the mixing range between the asthenospheric components and the subduction modified lithospheric component.
Volumme 326, pages 234-252,1 June 2022
Decoupled Zn-Sr-Nd isotopic composition of continental intraplate basalts caused by two-stage melting process.
Rong Xu, Yongsheng Liu, Sarah Lambart, Kaj Hoernle, Yangtao Zhu, Zongqi Zou, Junbo Zhang, Zaicong Wang, Ming Li, Frederic Moynier, Keqing Zong, Haihong Chen, Zhaochu Hu
Ocean island basalts (OIBs) with Zn isotopic ratios higher than the normal mantle (δ66Zn = 0.17 ± 0.08‰) or mid-ocean ridge basalts (MORBs; δ66Zn = 0.27 ± 0.06‰) generally also have an enriched Sr-Nd isotopic signature, suggesting carbonate-bearing eclogites, whose protolith is inferred to be subducting altered oceanic crust, in their mantle source. On the contrary, continental intraplate basalts with high δ66Zn usually show depleted Sr-Nd isotopic signatures (i.e., decoupled Zn-Sr-Nd isotopic composition). To elucidate the origin of the decoupled Zn-Sr-Nd isotopic composition in continental intraplate basalts, we report the discovery of both coupled and decoupled Zn-Sr-Nd isotopic data for a suite of Cenozoic continental intraplate basalts from the Zhejiang province, Southeast China. These basalts display clear spatial and temporal geochemical variations, with early-stage inland low-silica samples presenting moderately enriched Sr-Nd isotopic signatures and high δ66Zn (coupled Zn-Sr-Nd isotopic composition, similar to OIBs), and later-stage coastal high-silica samples that display a pronounced δ66Zn decrease with increasing SiO2 and 87Sr/86Sr and with decreasing alkali contents and 143Nd/144Nd (decoupled Zn-Sr-Nd isotopic composition). The early-stage basalts with coupled high Zn-Sr-Nd isotopic signatures are also more enriched in incompatible elements than any other basalts from eastern China reported so far. We explain the spatial and temporal geochemical variations of these basalts as the result of two main melting events: 1) the low-silica early-stage magmatism mostly occurs inland and results from high-pressure partial melting of a carbonated eclogite-bearing asthenospheric mantle. Because of the presence of a thick lithosphere limits the melting of the depleted mantle component, the signature of the Zn-Sr-Nd isotopically enriched, and more fusible carbonated eclogite is preserved. 2) At the later stage, magmatism mostly occurs on the coast where the subcontinental lithosphere is thinner. Hence, decompression melting progresses to shallower depth, resulting in an increase of the contribution from the depleted peridotite matrix and a dilution of the signal from the isotopically enriched fusible component. Further upwelling and in-situ melting at the base of the subduction-modified sub-continental lithospheric mantle (SCLM) explains both the decoupled Zn-Sr-Nd isotopic signature of the coastal basalts and their major and trace element variability. We further propose that decompression melting is driven by small-scale convection resulting from variations of lithospheric thickness. Our data highlight the importance of dynamic melting of carbonated eclogite-bearing asthenosphere and subsequent lithospheric melting in preservation and destruction of the coupled enriched Zn-Sr-Nd isotopic signature of carbonated eclogite component and generation of the apparent decoupled Zn-Sr-Nd isotopic signal commonly observed in continental intraplate basalts.
Geophysical Monograph Series
Volumme 263, Chapter 6, 11 june 2021
In: Konter J., Ballmer M, Cottaar S, & Marquardt H. (Eds. ), Mantle Convection and Surface Expressions.
Invited Contribution
Plain Language Summary:
Volcanic rocks on Earth share a common history – melting in the mantle tens to hundreds of kilometers deep, traveling through the mantle to the crust, and cooling and partial crystallizing in the crust, before finally reaching the surface – and yet, they show an incredible compositional variability. What creates such variability? In this chapter, we discuss the potential causes by looking at the role of the heterogeneities in the mantle and the fate of the magmas along their journey to the surface through the mantle and the crust. The discussion is based on experimental studies and natural compositions of about 60,000 volcanic rocks, melt inclusions (small pools of magma trapped inside the minerals) and cumulates (products of the crystallization of the magmas) from three main geological settings: divergent plate boundaries (mid-ocean ridges), intra tectonic plates (oceanic islands) and convergent plate boundaries (subduction zones).
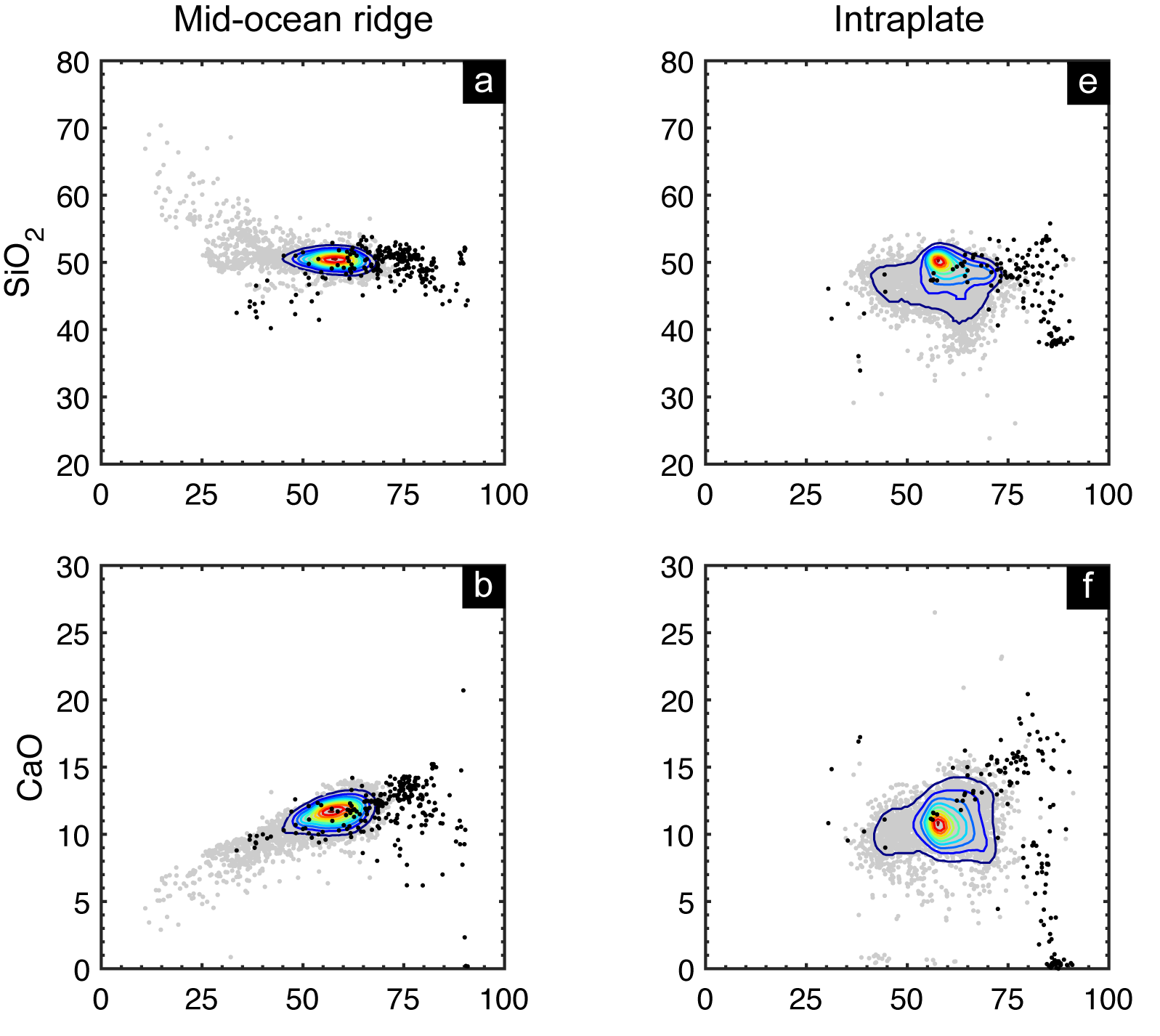
SiO2 and CaO contents vs. Mg# for lavas (gray circles) and cumulates (black) collected at mid-ocean ridges and on oceanic islands. Colored curves show the density contours (at intervals of 10%) for volcanic rocks.
Earth Science Reviews
Volume 207, August 2020, 103253
Abstract:
Large igneous provinces on Earth result from anomalously enormous volcanic eruptions at high melt production rates. These eruptions are often linked to catastrophic events such as mass extinctions, global climate changes, or continental break-up. Decoding their petrogenesis is therefore of great importance for our comprehensive understanding of the evolution and geodynamics of our planet. The ~260 Ma Emeishan large igneous province is an important geological feature of SW China with world-class ore deposits and is also suggested to be linked with the Capitanian mass extinction. However, fundamental aspects of the genesis of Emeishan province's most primitive lavas (picrites), such as the source lithology (pyroxenite or peridotite), the origin of compositional variations of olivines and the melting temperature and pressure conditions, remain poorly constrained. Here, we compile information on melt inclusion and host olivine, and whole-rock compositions from the ELIP picrites and show that these data are consistent with decompression melting of a relatively homogeneous peridotitic mantle plume, with a potential temperature higher than 1560 °C. The compositional variability of the olivines and picrites can be explained by varying the equilibrium depth of primary magma segregation and does not require the contribution of a pyroxenite component as previously suggested. Our results favor a scenario for the origin of the Emeishan large igneous province in which the decompression melting during upwelling of a hot hydrous and oxidized mantle plume is accompanied by catastrophic lithospheric thinning. In combination with the now extensive multi-element geochemical data, our findings provide a starting point for re-evaluation of the petrogenesis models for large igneous provinces.
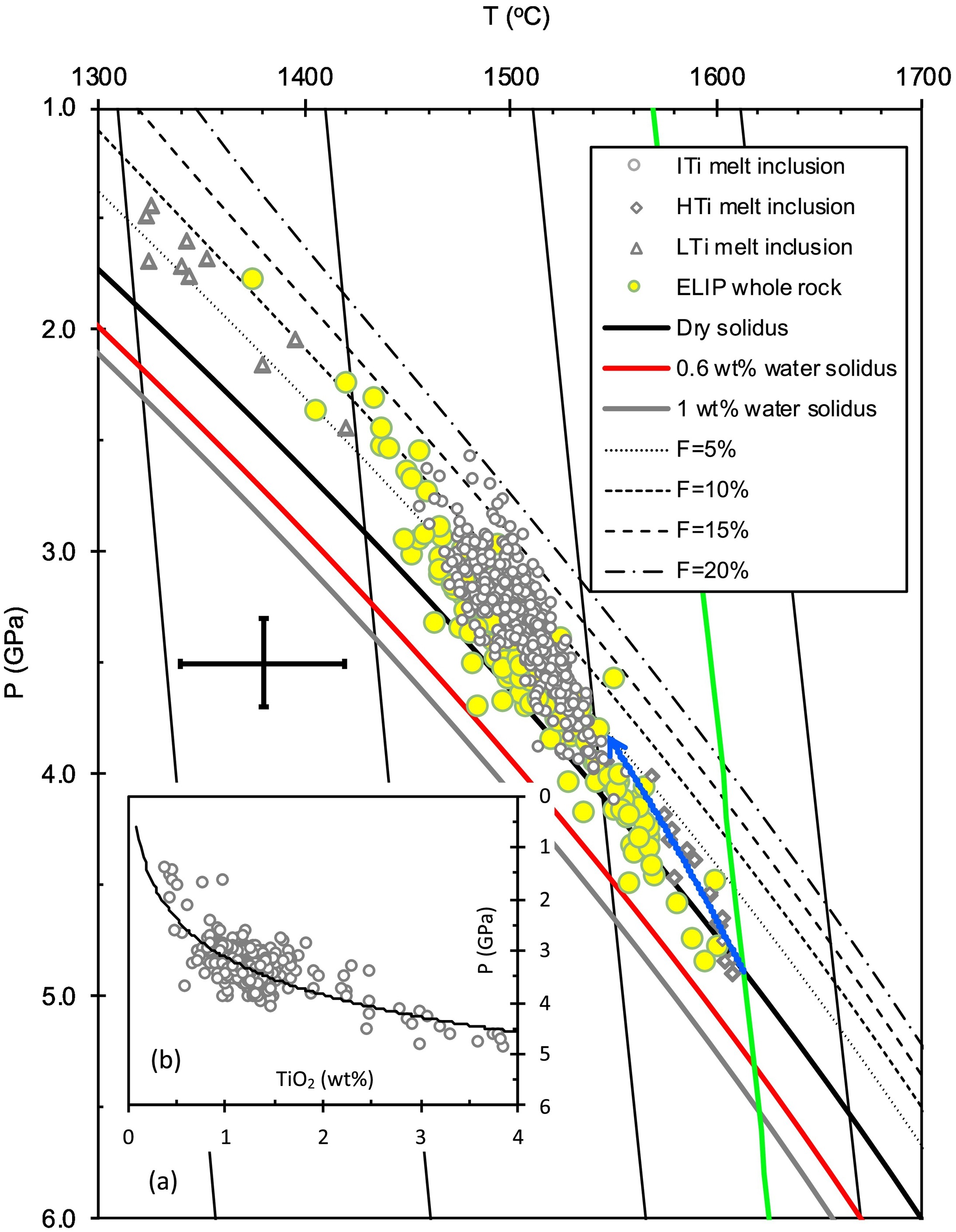
Melting P-T conditions underneath the ELIP. The curved blue line represents the possible melting adiabat, which is roughly fitted by the array of P-T data of ELIP picrites. This array intersects the anhydrous peridotite solidus at ~5 GPa, giving a minimal Tp of ~1560 °C (green adiabat).

Nature Geoscience
Volume 12, pages 482–486, 20 May 2019
Abstract:
The Earth’s mantle is heterogeneous as a result of early planetary differentiation and subsequent crustal recycling during plate tectonics. Radiogenic isotope signatures of mid-ocean ridge basalts have been used for decades to map mantle composition, defining the depleted mantle endmember. These lavas, however, homogenize via magma mixing and may not capture the full chemical variability of their mantle source. Here, we show that the depleted mantle is significantly more heterogeneous than previously inferred from the compositions of lavas at the surface, extending to highly enriched compositions. We perform high-spatial-resolution isotopic analyses on clinopyroxene and plagioclase from lower crustal gabbros drilled on a depleted ridge segment of the northern Mid-Atlantic Ridge. These primitive cumulate minerals record nearly the full heterogeneity observed along the northern Mid-Atlantic Ridge, including hotspots. Our results demonstrate that substantial mantle heterogeneity is concealed in the lower oceanic crust and that melts derived from distinct mantle components can be delivered to the lower crust on a centimetre scale. These findings provide a starting point for re-evaluation of models of plate recycling, mantle convection and melt transport in the mantle and the crust.
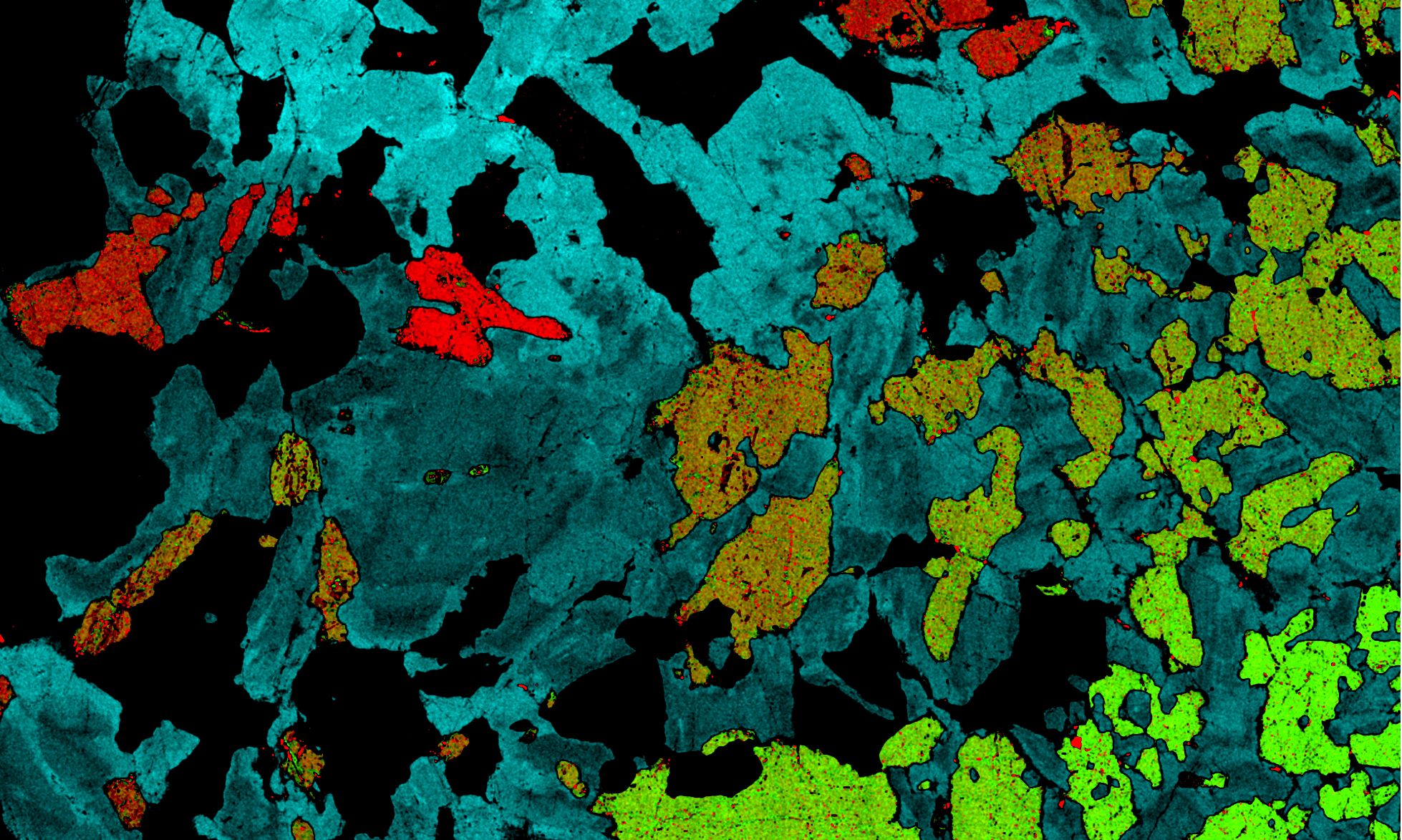
4cm-wide element map of an olivine gabbro; plagioclases in shades of blue, olivines in red to green; clinopyroxenes in black.

Lithos
Volumes 332–333, Pages 226-244, May 2019
Invited Review Article
Abstract:
Geochemically enriched signatures in global oceanic basalts have long indicated a heterogeneous mantle source, but the role of lithologic heterogeneity in producing mantle partial melts, particularly fertile pyroxenite rocks, remains unclear. Uranium-series disequilibria in basalts are particularly sensitive to the increased garnet mode and melting rates of pyroxenite rocks, making the system a useful indicator of mantle lithologic heterogeneity in the melt region for oceanic basalts. Here we summarize evidence for the presence and importance of pyroxenite rocks in the upper mantle and their role in melt generation of mid-ocean ridge basalts and ocean island basalts, with a synthesis of U-series disequilibrium systematics in oceanic basalts and implications for global lithologic heterogeneity of the upper mantle. We further synthesize the melt modeling approaches for the interpretation of U-series disequilibria in basalts and demonstrate the use of numerical solution models for time-dependent reactive porous flow and dynamic melting during decompression of a two-lithology mantle in thermal equilibrium. Our model outcomes corroborate prior interpretations in favor of reactive porous flow and two-porosity transport for relatively homogeneous, peridotite-dominated mantle regimes, and further supports contributions of pyroxenite partial melts to aggregated melts in order to reproduce the heterogeneous global basalt data. To most accurately predict the conditions of melting by comparison with measured data, two-lithology melting calculations should carefully consider the role of thermal equilibrium, mineral/melt partitioning, non-linear variations in mineral modes, and degree of melting during the melting process.
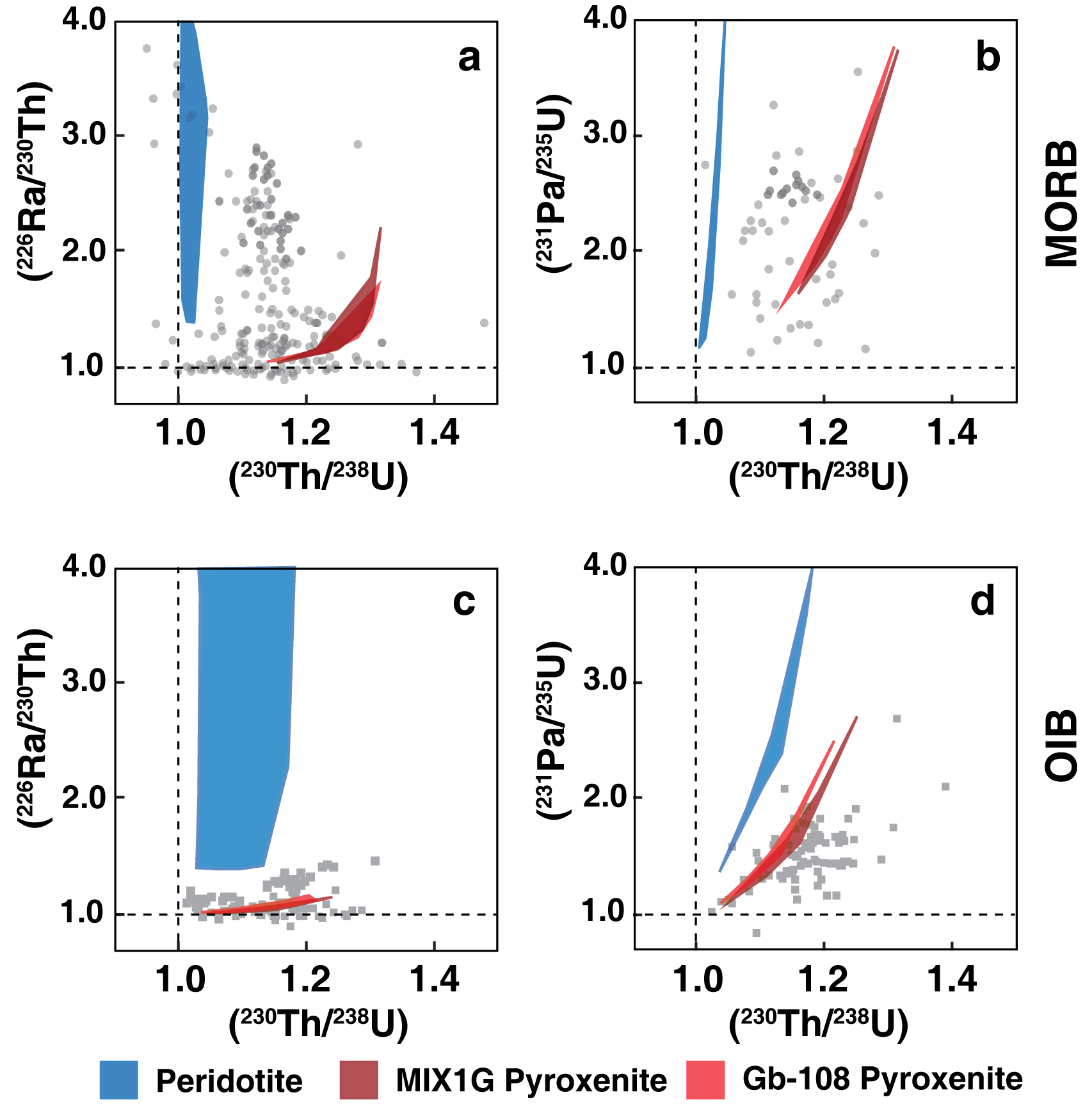
Melt modeling calculations for peridotite (blue fields), Gb-108 pyroxenite (red fields), and MIX1G pyroxenite (maroon fields) for scenarios relevant to MORB and OIB generation compared with global MORB (a-b) and OIB (c-d) data.
Geochemistry, Geophysics, Geosystems
Volume 19, Issue 9, Pages 3448-3458, September 2018
Abstract:
Mineral hydration and carbonation can produce large solid volume increases, deviatoric stress and fracture, that in turn can maintain or enhance permeability and reactive surface area. Despite the potential importance of this process, our basic physical understanding of the conditions under which a given reaction will drive fracture (if at all) is relatively limited. Our hydration experiments on CaO under uniaxial loads of 0.1 to 27 MPa show that strain and strain rate are proportional to the square root of time and exhibit negative, power-law dependence on uniaxial load, suggesting that (1) fluid transport via capillary flow is rate limiting and (2) decreasing strain rate with increasing confining pressure might be a limiting factor in reaction driven cracking at depth. However, our experiments also demonstrate that crystallization pressure due to hydration exceeds 27 MPa (consistent with a maximum crystallization pressure of 153 MPa for the same reaction, Wolterbeek et al., 2017). As a result, full hydration can be achieved at crustal depths exceeding 1km, which is relevant for engineered fracture systems.
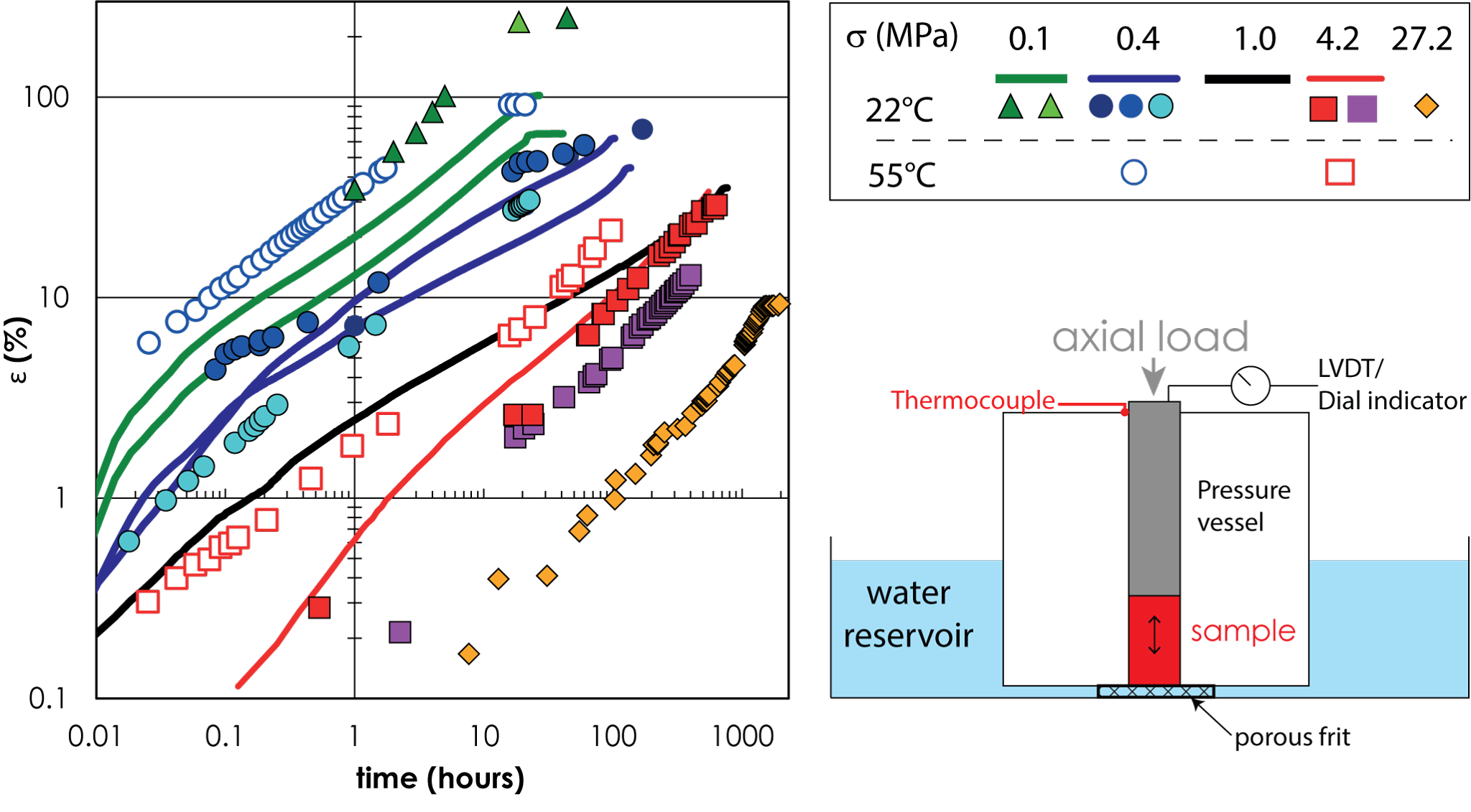
Left: Evolution of the volumetric strain, ε =ΔV/V as a function of time under uniaxial loads, σ, between 0.1 and 27.2 MPa. Right: Schematic of the experiments.
Energy Procedia
Volume 146, pages 92-102, 12 July 2018
Abstract:
In this invited review, we summarize the main results of ongoing research on “in situ” carbon mineralization in ultramafic rocks, including outcrop studies in Oman, investigation of carbon mass transfer in subduction zones from the Oman Drilling Project, laboratory investigations and numerical modeling of the pressure of crystallization and reaction-driven cracking, and assessment of the rate, cost and capacity of various proposed methods for engineered carbon mineralization.

White carbonate veins in partially serpentinized peridotites in Oman.
Geochemical Perspectives Letters
Volume 4, pages 7-12, 12 July 2017
Abstract:
Using Melt-PX to model the decompression melting of a heterogeneous mantle, I investigated the role of major-element composition of the lithologies present in the source on magmatic productivity, and trace element and isotopic melt compositions, independently of the bulk mantle composition. My calculations demonstrate that the volume of magma produced is not significantly affected by the nature of the lithological heterogeneity, but depends on the bulk mantle composition. However, an isochemical bulk mantle can produce contrasting trace element and isotopic melt compositions depending on the major-element compositions of the lithologies present in the source. Results show that the observed crust thickness of the Icelandic rift zones is consistent with about 10 % of recycled crust in the source, but also demonstrate there is no need to involve the contribution of melts derived from a recycled basalt component to explain the compositional variability of the Icelandic basalts in rift zones, and rather advance the contribution of olivine-bearing hybrid lithologies formed by solid-state reactions between the recycled crust and the peridotite.
Representation of the melting column in the three configurations. Colors show the lithologies that are partially melting at a given pressure.

Journal of Geophysical Research - Solid Earth
Volume 121 (8), pages 5708–5735, 18 August 2016
Abstract:
Geochemical and isotopic data suggest that the source regions of oceanic basalts may contain pyroxenite in addition to peridotite. In order to incorporate the wide range of compositions and melting behaviors of pyroxenites into mantle melting models, we have developed a new parameterization, Melt-PX, which predicts near-solidus temperatures and extents of melting as a function of temperature and pressure for mantle pyroxenites. We used 183 high-pressure experiments (25 compositions; 0.9–5 GPa; 1150–1675°C) to constrain a model of melt fraction vs. temperature from 5% melting up to the disappearance of clinopyroxene for pyroxenites as a function of pressure, temperature, and bulk composition. When applied to the global set of experimental data, our model reproduces the experimental F-values with a standard error of estimate of 13% absolute; temperatures at which the pyroxenite is 5% molten are reproduced with a standard error of estimate of 30°C over a temperature range of ~500°C and a pressure range of ~4 GPa. In conjunction with parameterizations of peridotite melting, Melt-PX can be used to model the partial melting of multi-lithologic mantle sources—including the effects of varying the composition and the modal proportion of pyroxenite in such source regions. Examples of such applications include calculations of isentropic decompression melting of a mixed peridotite + pyroxenite mantle; these show that, although the potential temperature of the upwelling mantle plays an important role in defining the extent of magma production, the composition and mass fraction of the pyroxenite also exert strong controls.
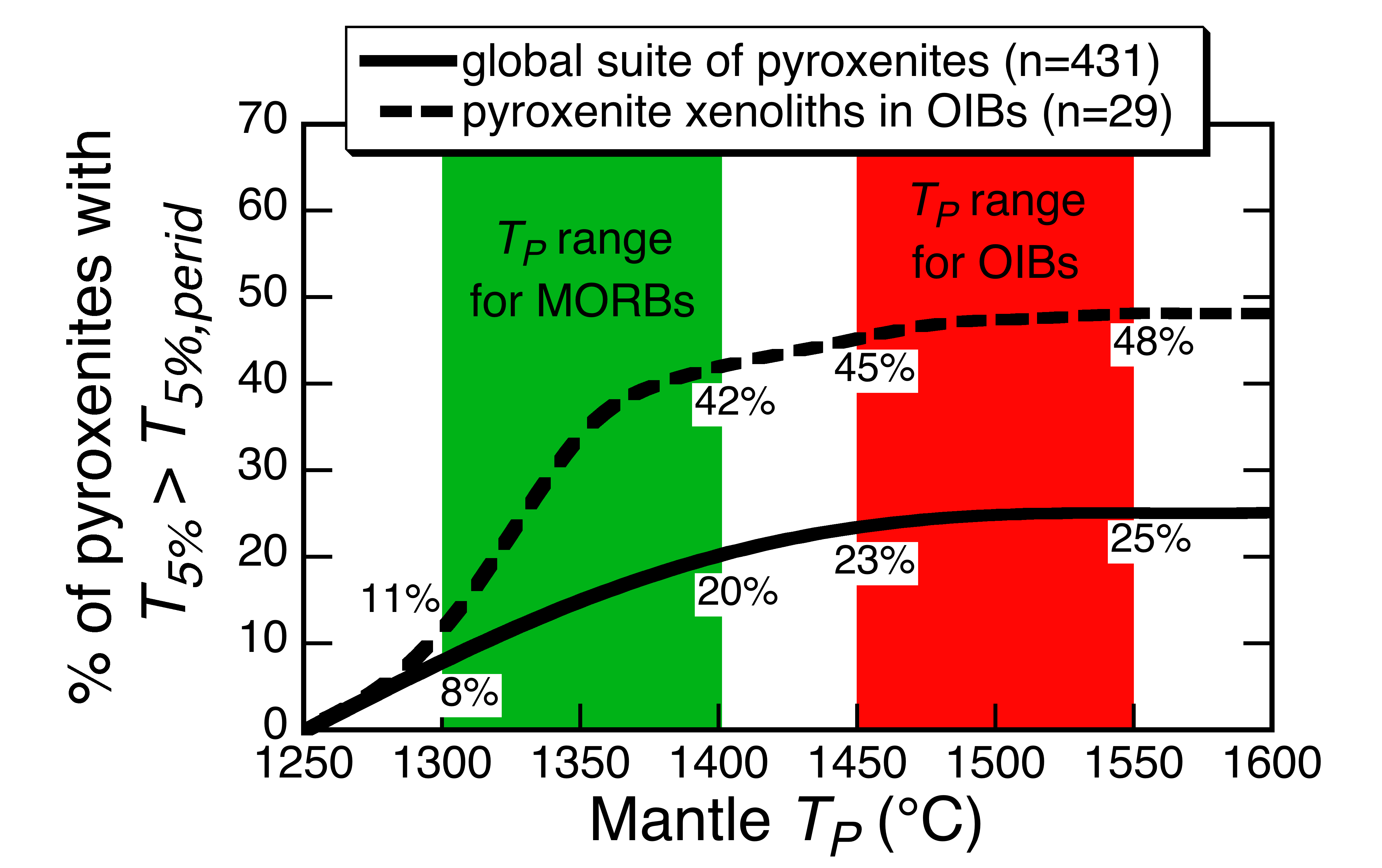
Percentage of natural pyroxenite compositions [Lambart et al., 2009a and references therein] that have calculated T5% (Melt-PX) > T5%,peridotite [Katz et al., 2003] as a function of mantle potential temperature (TP) (see text for details). Green and red vertical bands denote the TP ranges for MORB and OIB, respectively, [Courtier et al., 2007]; values at the boundaries of the bands denote pyroxenite percentages with T5% > T5%,peridotite.
Earth and Planetary Science Letters
Volume 404, pages 319-331, 15 October 2014
Abstract:
Piston-cylinder experiments were performed to characterize the composition of liquids formed at very low degrees of melting of two fertile lherzolite compositions with 430 ppm and 910 ppm K2O at 1 and 1.3 GPa. We used the microdike technique (Laporte D. et al., 2004. Contrib. Mineral. Petrol. 146: 463-484) to extract the liquid phase from the partially molten peridotite, allowing us to analyze liquid compositions at degrees of melting F down to 0.9 %. At 1.3 GPa, the liquid is in equilibrium with olivine + orthopyroxene + clinopyroxene + spinel in all the experiments; at 1 GPa, plagioclase is present in addition to these four mineral phases up to about 5 % of melting (T = 1240 °C). Important variations of liquid compositions are observed with decreasing temperature, including strong increases in SiO2, Na2O, K2O, and Al2O3 concentrations, and decreases in MgO, FeO, and CaO concentrations. The most extreme liquid compositions are phonolites with 57 % SiO2, 20-22 % Al2O3, Na2O + K2O up to 14 %, and concentrations of MgO, FeO, and CaO as low as 2-3 %. Reversal experiments confirm that low degree melts of a fertile lherzolite have phonolitic compositions, and pMELTS calculations show that the amount of phonolite liquid generated increases from 0.3 % in a source with 100 ppm K2O to more than 3 % in a source with 2000 ppm K2O. The enrichment in silica and alkalis with decreasing melt fraction results in major changes in melt structure and polymerization, which have important consequences for the partitioning of minor and trace elements. Thus Ti4+ in our experiments, and by analogy other highly charged cations and rare earth elements, become more compatible near the peridotite solidus. The generation of phonolite liquids by low degree partial melting of a fertile peridotite brings a strong support to the hypothesis that some phonolitic lavas or their plutonic equivalents (nepheline syenites) are produced directly by partial melting of mantle peridotites. The circulation of peridotite low-degree melts into the lithospheric mantle may be responsible for a special kind of metasomatism characterized by Si- and K-enrichment. If they are unable to escape by porous flow, low-degree melts will ultimately be trapped inside neighbouring olivine grains and give rise to the silica- and alkali-rich glass inclusions found in peridotite xenoliths.
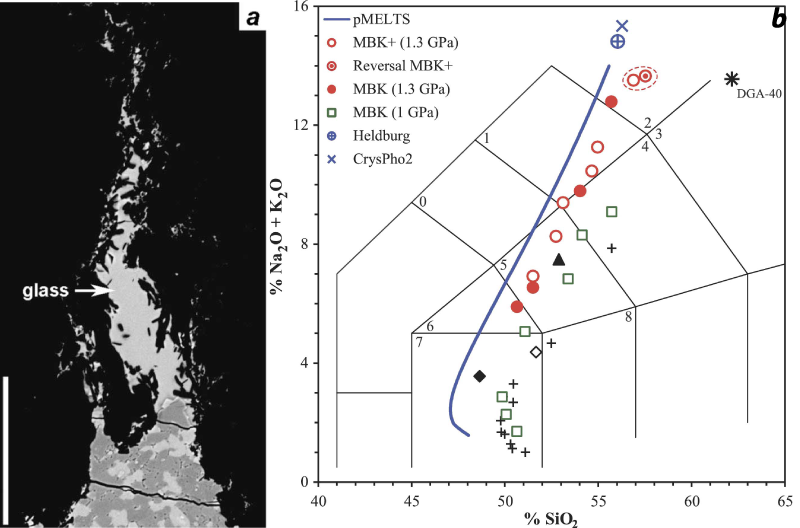
(a) SEM-Backscattered electron microphotograph of sample MBK3 (1.3 GPa-1200 °C) showing a microdike filled with quenched melt (“glass”) into the graphite container (black). (b) Composition of partial melts of fertile peridotites between 1 and 1.5 GPa plotted in the total alkali-silica diagram. Our partial melting experiments at 1.3 GPa are shown by the red circles (open circles: MBK+; solid circles: MBK); the open green squares are for the experiments with MBK at 1 GPa. The open red circle with a central dot is reversal experiment MBK+13: partial melting experiment MBK+6 and its reversal MBK+13 are surrounded by the red dashed line. The small black crosses correspond to fertile peridotite MM3 at 1 GPa; the diamonds correspond to a K2O-free analogue of MM3 at 1 and 1.5 GPa (open and solid diamonds, respectively). The solid triangle is the near-solidus melt of MORB-pyrolite MPY at 1.5 GPa. The blue symbols correspond to Heldburg phonolite and to the glass analyzed in the crystallization experiment CrysPho2 (crystallization of a composition equivalent to partial melt MBK+6 at 1 GPa-1150 °C). The star labeled “DGA-40” corresponds to a trachyte melt in equilibrium with ol + opx + cpx at 1.2 GPa and 1150 °C. Melt compositions computed by pMELTS for MBK+ at 1.3 GPa are shown by the blue curve (the theoretical trend is shifted to the left of the experimental data because of a slight underestimation of SiO2 by pMELTS). The grid corresponds to the chemical classification of volcanic rocks, with the main fields of interest labeled as follows: (0) Phonotephrite; (1) Tephriphonolite; (2) Phonolite; (3) Trachyte; (4) Trachyandesite; (5) Basaltic trachyandesite; (6) Trachybasalt; (7) Basalt; (8) Basaltic andesite.
Earth and Planetary Science Letters
Volume 395, pages 24-40, 1 June 2014
Abstract:
We present a method that can be used to estimate the amount of recycled material present in the source region of mid-ocean ridge basalts by combining three key constraints: (1) the melting behaviour of the lithologies identified to be present in a mantle source, (2) the overall volume of melt production, and (3) the proportion of melt production attributable to melting of each lithology.
These constraints are unified in a three-lithology melting model containing lherzolite, pyroxenite and harzburgite, representative products of mantle differentiation, to quantify their abundance in igneous source regions.
As a case study we apply this method to Iceland, a location with sufficient geochemical and geophysical data to meet the required observational constraints. We find that to generate the 20 km of igneous crustal thickness at Iceland coasts, with 30 ± 10% of the crust produced from melting a pyroxenitic lithology, requires an excess mantle potential temperature (ΔTp) of ∼ 130°C (Tp ∼ 1460°C) and a source consisting of at least 5% recycled basalt. Therefore, even with lithological heterogeneity the mantle beneath Iceland requires a significant excess temperature to match geophysical and geochemical observations: lithological variation alone is not viable. Determining a unique source solution is only possible if mantle potential temperature is known precisely and independently, otherwise a family of possible lithology mixtures is obtained across the range of viable ΔTp. For Iceland this uncertainty in ΔTp means that the mantle could be > 20% harzburgitic if ΔTp > = 150°C (Tp > = 1480°C).
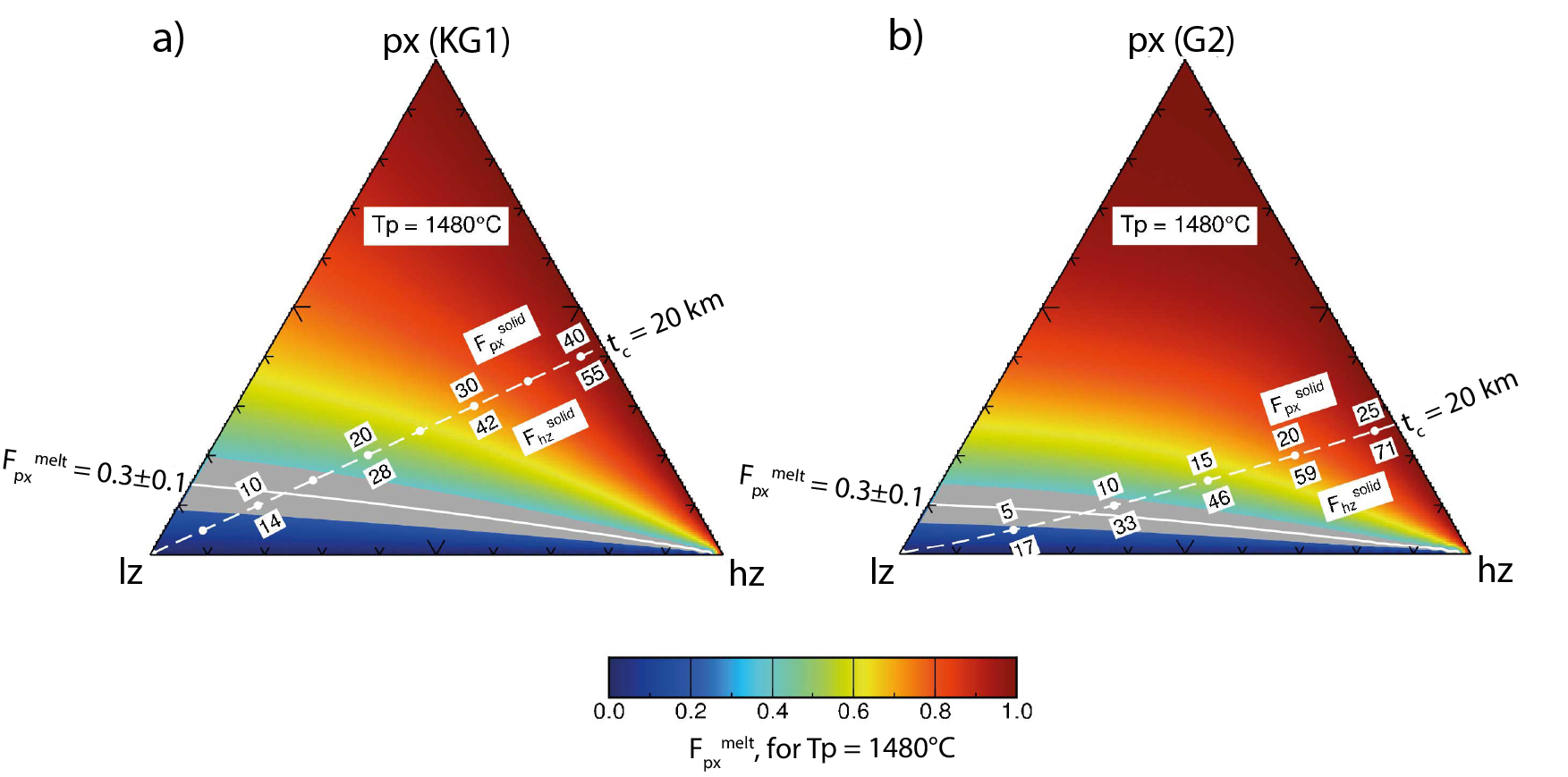
Ternary diagrams combining crustal thickness (tc) and geochemical constraints (Fpxmelt) to identify the allowable lithology combinations beneath Iceland. Each apex of the ternary represents an endmember lithology in the mantle: lherzolite (lz), harzburgite (hz) and pyroxenite (px, for (a) the KG1 composition from Kogiso et al., 1998, for (b) the G2 composition from Pertermann and Hirschmann, 2003). The three endmember lithologies are mechanically mixed in variable proportions and three-lithology melting calculations performed to fill in the ternary space for Fpxmelt and tc. Background colours in these diagrams correspond to Fpxmelt determined for model runs with Tp = 1480 °C. The dashed white line marks the lithology combinations melting to produce a tc = 20 km, the solid white line and grey shaded region mark the lithology combinations reproducing the observed Fpxmelt. The point of intersection of the solid and dashed white lines is the lithology mixture able to match both crustal thickness and geochemical constraints (in (a) this is lz71hz17px12, in (b) lz70hz22px8).
Lithos
Volume 160-161, pages 14-36, February 2013
Invited Review Article
Abstract:
Based on previous and new results on partial melting experiments of pyroxenites at high pressure, we attempt to identify the major element signature of pyroxenite partial melts and to evaluate to what extent this signature can be transmitted to the basalts erupted at oceanic islands and mid-ocean ridges. Although peridotite is the dominant source lithology in the Earth's upper mantle, the ubiquity of pyroxenites in mantle xenoliths and in ultramafic massifs, and the isotopic and trace elements variability of oceanic basalts suggest that these lithologies could significantly contribute to the generation of basaltic magmas. The question is how and to which degree the melting of pyroxenites can impact the major-element composition of oceanic basalts. The review of experimental phase equilibria of pyroxenites shows that the thermal divide, defined by the aluminous pyroxene plane, separates silica-excess pyroxenites (SE pyroxenites) on the right side and silica-deficient pyroxenites (SD pyroxenites) on the left side. It therefore controls the melting phase relations of pyroxenites at high pressure but, the pressure at which the thermal divide becomes effective, depends on the bulk composition; partial melt compositions of pyroxenites are strongly influenced by non-CMAS elements (especially FeO, TiO2, Na2O and K2O) and show a progressive transition from the liquids derived from the most silica-deficient compositions to the liquids derived from the most silica-excess compositions.
Another important aspect for the identification of source lithology is that, at identical pressure and temperature conditions, many pyroxenites produce melts that are quite similar to peridotite-derived melts, making difficult the determination of the presence of pyroxenite in the source regions of oceanic basalts; only pyroxenites able to produce melts with low SiO2 and high FeO contents can be identified on the basis of the major-element compositions of basalts. In the case of oceanic island basalts, a high CaO/Al2O3 ratio can also reveal the presence of pyroxenite in the source-regions. Experimental and thermodynamical observations also suggest that the interactions between pyroxenite-derived melts and host peridotites play a crucial role in the genesis of oceanic basalts by generating a wide range of pyroxenites in the upper mantle: partial melting of such secondary pyroxenites is able to reproduce the features of primitive basalts, especially their high MgO contents, and to transmit, at least in some cases, the major-element signature of the original pyroxenite melt to the oceanic basalts. At last, we highlight that the very silica depleted compositions (SiO2 > 42 wt%) and high TiO2 contents of some OIBs seem to require the contribution of fluids (CO2 or H2O) through melting of either carbonated lithologies (peridotite or pyroxenite) or amphibole-rich veins.
(a) Iron versus MgO contents (in wt.%) of natural pyroxenites (small grey circles) and hornblendites (pink stars) from xenoliths and alpine-type massifs compared to starting compositions in experimental studies (large circles). Pyroxenites M5‐40 and M7‐16 used as starting materials in this study are represented by the red and purple circles, respectively. Also shown are the fields of mantle peridotites (green area) and MORB matrix glasses (yellow area). (b)Total iron contents versus SiO2 contents for MORB glasses with MgO ≥ 9 wt.%. Red symbols represent MORB glasses with Mg# ≥ 67. The orange rectangle represents the compositional area of px‐MORBs (with both lower SiO2 and higher FeOT than MORB glasses with Mg# ≥ 67), good candidates to carry the signature of SiO2-poor and FeO-rich pyroxenites, such as M7-16.
Journal of Petrology
Volume 53(3), pages 451-476, March 2012
Abstract:
We performed a thermodynamic and experimental study on the fate of pyroxenite-derived melts during their migration through the peridotitic mantle. We used a simplified model of interaction, where peridotite is impregnated by and then equilibrated with a finite amount of pyroxenite-derived liquid. We considered two pyroxenite compositions and three contexts of pyroxenitic melt impregnation: (i) in a subsolidus lithospheric mantle, (ii) beneath a mid-ocean ridge (MOR) in a subsolidus asthenospheric mantle at high pressure, and (iii) beneath MOR in a partially molten asthenospheric mantle. Calculations were performed with pMELTS at constant pressure and temperature with a melt-rock ratio varying from 0 to 1. Concurrently, a series of impregnation experiments was performed at 1 and 1.5 GPa to reproduce the final stages of calculations where the magma-rock ratio is 1.
Incoming melt and host rocks react differently according to melt composition and the physical state of the surrounding mantle. Whereas clinopyroxene (Cpx) is systematically a reaction product, the role of olivine (Ol) and orthopyroxene (Opx) depends on incoming melt silica activity aSiO2: if it is lower than the silica activity a0SiO2 of a melt saturated in Ol and Opx at the same pressure P and temperature T, Opx is dissolved and Ol precipitates, and conversely if aSiO2 gt; a0SiO2 (see figure). Such contrasted reactions between pyroxenitic melts and peridotitic mantle may generate a large range of new lithological heterogeneities (wehrlite, websterite, clinopyroxenite) in the upper mantle. Also, our study shows that the ability of pyroxenite-derived melts to migrate through the mantle depends on the melting degree of surrounding peridotite: the reaction of these melts with a subsolidus mantle results in a strong melt consumption (40-100%) and large Cpx production (with some spinel or garnet, depending on P). This is expected to drastically decrease the system permeability and the capacity of pyroxenite-derived melts to infiltrate neighbouring rocks. On the contrary, melt migration to the surface should be possible if the surrounding mantle is partially melted: though liquid reactivity varies with composition, melt consumption is then restricted to less than 20%. Hence, magma/rock interactions can have a significant impact on the dynamics of melting and magma migration and should not be neglected when modelling the partial melting of heterogeneous mantle.
Sketch of MORB petrogenesis in the case of a heterogeneous mantle composed of pyroxenite veins (stippled, folded layers at the bottom) in a peridotite matrix. Processes acting at a given depth are listed in the boxes on the right: melting processes in red boxes, and peridotite-pyroxenite interactions in yellow boxes. The outcome of each process for the chemical and mineralogical evolution of mantle is summarized in the left column. The grey triangle is the melting zone: in its lower part, only pyroxenites are partially molten; in its upper part (hatched area), both pyroxenites and peridotites are partially molten. Black arrows above the peridotite solidus mark the onset of penetrative porous flow; white arrows represent melt focusing into high-permeability channels.
Earth and Planetary Science Letters
Volume 288(1-2), pages 335-347, 30 October 2009
Abstract:
To better assess the potential role of pyroxenites in basalt generation at mid-ocean ridges, we performed partial melting experiments on two natural websterites and one clinopyroxenite representative of worldwide pyroxenites. The experiments were conducted at 1 and 1.5 GPa in a piston-cylinder apparatus; the microdike technique was used to separate the liquid fromthe solid phases and to obtain reliable glass analyses even at low degrees of melting. Contrasted melting behaviors were observed depending on the phase proportions at the solidus, especially the abundance of orthopyroxene. (1) If orthopyroxene is abundant, the main melting reaction is similar to the melting reaction in peridotites (clinopyroxene+orthopyroxene±spinel=liquid+olivine), and the liquids are similar to peridotite-derived melts for most major elements. (2) In the absence of orthopyroxene, the mainmelting reaction is clinopyroxene+spinel=liquid+olivine, yielding liquids that are strongly depleted in SiO2 in comparison to peridotite-derived melts. This low-SiO2 content can be associated with a high FeO content, a combination usually ascribed to a high average pressure of melting (of a peridotitic source).
Because of their higher melt productivities and lower solidus temperatures, 5wt.% of pyroxenites in a heterogeneous mantle may contribute up to 40wt.% of the total melt production. (1) In some cases, pyroxenitederived melts differ strongly from peridotite partial melts, leading to a distinct pyroxenite signature in the average melt (lower alkali and TiO2 contents, lower SiO2, higher FeO and/or lower Mg#). The classical criteria used to select primitive mantle-derived magmas (melt inclusions hosted into highMg# olivine orMORB glasses with Mg# ≥67) or to track down enriched mantle sources (MORB glasses with high incompatible element contents) must be considered with caution, otherwise melts carrying a pyroxenite signature may be eliminated. (2) In general, however, the major-element signature of pyroxenites should be hardly detectable in the average melt because of the similarity of most pyroxenite-derived melts with peridotite partial melts. This similarity may explain why MORB have relatively uniform major-element compositions, but may have variable trace element and/or isotopic compositions.
Melt compositions in pyroxenites M5–103, M5–40, and M7–16 at 1 GPa plotted as a function of temperature.
The temperature interval of 1245–1305 °C represented by yellow boxes corresponds to the range of temperatures (at 1 GPa) of a mantle undergoing adiabatic decompression melting (assuming potential temperatures in the range 1280–1400 °C, McKenzie and Bickle (1988)). Symbols are as follows: diamonds, M5–103; triangles, M5–40; squares, M7–16. Green fields correspond to liquids produced by peridotites PHN1611, MM3, DMM1 and Depma. Error bars (1σ on oxide concentrations; ±5 °C on temperature) are shown in the bottom right corner of each diagram.
Main result from this study: (1) Most of pyroxenites produce liquids that are similar to peridotite-derived melts for most major elements (SiO2, Al2O3, CaO, MgO, and FeO). This may explain why MORB have relatively uniform major-element compositions, but may have variable trace element and isotopic compositions. (2) Pyroxenite melts are not enriched (and may even be depleted) in incompatible elements (Na2O, TiO2 and K2O) in comparison to peridotite-derived melts. Therefore, the concentrations of these elements cannot be used as markers of pyroxenites in MORB mantle sources. (3) Some pyroxenites yield melts with a distinct signature, such as a low-SiO2 content and/or a high FeO content, two features usually ascribed to a high average pressure of melting (of a peridotitic source).
Contributions to Mineralogy and Petrology
Volume 157(4), pages 429-451, April 2009
Abstract:
We performed experiments in a piston-cylinder apparatus to determine the effects of focused magma transport into highly permeable channels beneath midocean ridges on: (1) the chemical composition of the ascending basalt; and (2) the proportions and compositions of solid phases in the surrounding mantle. In our experiments, magma focusing was supposed to occur instantaneously at a pressure of 1.25 GPa. We first determined the equilibrium melt composition of a fertile mantle (FM) at 1.25 GPa-1310°C; this composition was then synthesised as a gel and added in various proportions to peridotite FM to simulate focusing factors X equal to 3 and 6 (X = 3 means that the total mass of liquid in the system increased by a factor of 3 due to focusing). Peridotite FM and the two basalt-enriched compositions were equilibrated at 1 GPa-1290°C; 0.75 GPa-1270°C; 0.5 GPa-1250°C, to monitor the evolution of phase proportions and compositions during adiabatic decompression melting. Our main results may be summarised as follows: (1) magma focusing induces major changes of the coefficients of the decompression melting reaction, in particular, a major increase of the rate of opx consumption, which lead to complete exhaustion of orthopyroxene (and clinopyroxene) and the formation of a dunitic residue.
A focusing factor of ≈4 (that is, a magma/rock ratios equal to ≈0.26) is sufficient to produce a dunite at 0.5 GPa. (2) Liquids in equilibrium with olivine (±spinel) at low pressure (0.5 GPa) have lower SiO2 concentrations, and higher concentrations in MgO, FeO, and incompatible elements (Na2O, K2O, TiO2) than liquids produced by decompression melting of the fertile mantle, and plot in the primitive MORB field in the olivine–silica–diopside–plagioclase tetrahedron. Our study confirms that there is a genetic relationship between focused magma transport, dunite bodies in the upper mantle, and the generation of primitive MORBs.
Simplfied model of decompression melting, magma focusing and transport beneath mid-ocean ridges
(1) Adiabatic decompression (TP = 1350°C)
(2) A single event of magma focusing is assumed to occur at P = 1.25 GPa and T = 1310°C, when the degree of melting is close to 10%: the total mass of partial melt in a "channel" is multiplied by a factor Ω.
(3) Adiabatic decompression from 1.25 GPa to 0.5 GPa.
(4) At 0.5 GPa, liquid is extracted from the solid matrix to form dykes.
(5) Rapid ascent of magmas in dykes to a shallow chamber beneath the mid ocean ridge.
Pictures are backscattered electron micrographs illustrating the textures and phase assemblages in the three experimental series before and after the focusing event. Scale bars: 10 μm
The opening of the northeast Atlantic, starting around 56 My ago, was associated with the emplacement of the North Atlantic Igneous Province, including the deposition of voluminous extrusive basaltic successions and intrusion of magma into the surrounding sedimentary basins. The mid-Norwegian Margin is a global type example of such a volcanic rifted margin and is well suited for scientific drilling with its thin sediment cover and good data coverage. During International Ocean Discovery Program Expedition 396, 21 boreholes were drilled at 10 sites in five different eological settings on the mid-Norwegian Margin. The boreholes sampled a wide variety of igneous and sedimentary settings ranging from lava flow fields to hydrothermal vent complexes, along with thick successions of Upper Paleocene and Lower Eocene strata. A comprehensive suite of wireline logs was collected in eight boreholes. These data provide new constraints for geodynamic models to explain the rapid emplacement of large igneous provinces and will also allow us to test the hypothesis that the Paleocene–Eocene Thermal Maximum (PETM) was caused by hydrothermal release of carbon in response to magmatic intrusions and/or flood basalt eruption. Successful drilling and high core recovery of target intervals at all nine primary sites and one additional alternate site will allow us to achieve these goals during postcruise work. Expedition 396 highlights include (1) drilling and coring a unique, multihole transect across a supra-sill hydrothermal system and crater that was filled in during the PETM, (2) drilling and coring all the major lithofacies at each of the component parts of a volcanic rifted margin from terrestrial to deep marine, and (3) acquiring excellent petrophysical data and imaging support for core analyses of complex and diverse volcanic and volcaniclastic intervals across the terrestrial to marine transition.
Proceedings of the IODP
Expedition #396
Published on April 6th, 2023
doi:10.14379/iodp.proc.396.2023
Expedition 396 summaryThe opening of the North Atlantic about 56 My ago was associated with the emplacement of the North Atlantic Igneous Province, including the deposition of voluminous extrusive basaltic successions and intrusion of magma into the surrounding sedimentary basins. The mid-Norwegian Margin is a global type example of such volcanic rifted margins and is well suited for scientific drilling with its thin sediment cover and good data coverage. During International Ocean Discovery Program Expedition 396, 21 boreholes were drilled at 10 sites in five different geological settings on this volcanic margin. The boreholes sampled a multitude of igneous and sedimentary settings ranging from lava flow fields to hydrothermal vent complexes, along with thick successions of upper Paleocene and lower Eocene strata. A comprehensive suite of wireline logs was collected in eight boreholes. The main goals of the expedition were to provide constraints for geodynamic models to test different hypotheses that can explain the rapid emplacement of large igneous provinces and the hypothesis that the associated Paleocene/Eocene Thermal Maximum was caused by hydrothermal release of carbon in response to magmatic intrusions. Successful drilling, combined with high core recovery of target intervals of all nine primary sites and one additional alternate site, should allow us to achieve these goals during postcruise work.
* Amar Agarwal, Graham D.M. Andrews, Peter Betlem, Joyeeta Bhattacharya, Henk Brinkhuis, Sayantani Chatterjee, Marialena Christopoulou, Vincent J. Clementi, Eric C. Ferré, Irina Y. Filina, Joost Frieling, Pengyuan Guo, Dustin T. Harper, Morgan T. Jones, Sarah Lambart, Jack Longman, John M. Millett, Geoffroy Mohn, Reina Nakaoka, Reed P. Scherer, Christian Tegner, Natalia Varela, Mengyuan Wang, Weimu Xu, Stacy L. Yager.
First Row Transition Elements (FRTEs) have been identified as potential petrogenetic indicators because of their contrasted partitioning behaviors between the solid mantle phases. Using a combination of published data on natural and experimental samples and new high precision analyses (high-current microprobe and LA-ICP-MS analyses), we investigate the various parameters that control FRTE partitioning between common mantle minerals. Our results show that mantle pyroxenites (found in ophiolites and ultramafic massifs) and peridotites share same mineralmineral exchange coefficients, Kdmnl1/mnl2, independently of their compositions as well as their pressures and temperatures of equilibration. Mn preferentially partitions in garnet (Kd(Mn/Fe)gt/cpx = 1.56 ± 0.54) rather than in orthopyroxene and olivine (0.699 ± 0.152 and 0.466 ± 0.131, respectively) in pyroxenites, with similar coefficient values for peridotites. On the contrary, Zn and Ni both preferentially partition in olivine rather than in clinopyroxene and garnet. Ni also shows a strong affinity with spinel (Kd(Ni/Mg)sp/ol ∼ 1.8-2.1), suggesting that even small proportions of spinel in mantle lithologies should be taken into account when modeling the partitioning behavior of Ni. Preliminary observations on eclogites (i.e., rocks resulting from the metamorphism of the oceanic crust) and arclogites (i.e., mafic cumulates found as xenoliths in arc settings), however, show that they present distinctively higher Kd(Mn/Fe)gt/cpx and lower Kd(Zn/Fe)gt/cpx than those in mantle lithologies, implying that convection and mixing into the asthenospheric mantle may significantly affect the partitioning behavior of FRTEs. Finally, we tested if we could use FRTE ratios from basalt compositions to solve for modal proportions in the mantle source. We applied the Kd determined from mantle lithologies in this study, along with experimental melt-mineral partitioning coefficients and a simple batch melting model, on two series of basalts from Iceland and the Reykjanes ridge segment (1,2) showing contrasted FRTE concentrations. Individual FRTE ratios do not provide unique solution. In fact, a same FRTE ratio can be explained by a range of modal proportions in the source. However, when combined, FRTE ratios can be used to better constrain the nature of the source. [1] Eason et al., 2015, Bull. Volcanol., doi:10.1007/s00445- 015-0916-0 [2] Jenner and O’Neill, 2012, Geochem. Geophys. Geosyst., doi:10.1029/2011GC003890

Oral presentation: 5 July
Abstract: 7709
Session 2e: Mantle heterogeneity: origins and contribution to magmatism and implications for mantle dynamics
In a classic model of evolution of the non-cratonic continental mantle lithosphere, harzburgites are refractory (<5% clinopyroxene) and represent the residue of partial melting of a fertile mantle composed of lherzolites (>5% clinopyroxene). However, partial melting is not the only process that could explain the peridotite compositional variability that ranges from fertile (>2wt. % Al2O3, <45wt. % MgO) to refractory (<2wt. % Al2O3, >45 wt. % MgO). In the refertilization process, harzburgite is a refractory protolith that undergoes reactive percolation of silicate melts, derived from the underlying asthenosphere and causing the crystallization of a new generation of minerals (mostly clinopyroxene). Here we use phMELTS [1,2] and a simple impregnation model to independently constrain the role of five different parameters, pressure, temperature, redox conditions, and compositions of the percolated rock and of the percolating melt (hereafter referred as P-T-fO2-X∏-Xmelt), during the refertilization process, and compare these results with observed suites of peridotites. The evolution of the modal composition, together with the major element variations, are evaluated. The main results are that first, the produced model is consistent with the global peridotite database, and second, T, fO2 and small variations of pressure have almost no impact on the evolution of the system. The percolated harzburgite modal composition has an effect on the variation of the modal proportions. The parameter with the most significant impact is Xmelt, which is directly linked to the geodynamic context and to melting conditions. This parameter directly controls the refertilization reaction and so, the phase composition. Incompatible element (e.g., Na) depletions in natural residual assemblages are lower than those predicted from the simple impregnation model, indicating instead that the process occurs in open systems, and that reactive percolation likely results in incompatible element enrichment in the associated melt. Our results corroborate that most of the spectrum of compositional variability observed in lithospheric mantle peridotites can be explained by the impregnation of primitive silicate melt in refractory harzburgites.

Flash talk: 5 July
Abstract: 5861
Session 2e: Mantle heterogeneity: origins and contribution to magmatism and implications for mantle dynamics
Mantle heterogeneity has a first-order control on the petrological and geochemical differences of erupted mafic lavas across the globe. It is debated whether this heterogeneity reflects only chemical variability or also lithological differences in source regions. Because of their various partitioning behaviors between mantle minerals, First Row Transition Elements (FRTEs) have been identified as potential lithological tracers. Here, we investigate the various parameters that control FRTE partitioning between common mantle phases through a comparison of partition coefficients calculated from natural pyroxenites obtained from the Earthchem database with previous partitioning experiments and new electron microprobe analyses. Using naturally occurring pyroxenites from alpine massifs and xenoliths provides the opportunity to explore the behavior of FRTEs on a much larger range of compositions and temperatures than covered by experimental studies. Our preliminary results show that natural partition coefficients for Fe and Mn depend on temperature and vary distinctly between lithologies. The effect of composition, however, is difficult to resolve and will require further inspection. Natural exchange coefficients, or Kd’s (mineral/mineral) for Mn/Fe, largely match previous experimental data across peridotite and pyroxenite compositions for garnet/clinopyroxene(cpx), orthopyroxene/cpx, and olivine/cpx. However, natural samples often present evidence of chemical disequilibrium and/or secondary alteration which can significantly increase the scatter in analyses. Importantly, despite the larger uncertainty on the natural Kd’s than on experimental ones, natural exchange coefficients show distinct values between the various pairs of minerals. These distinctions, and the fact that Kd’s do not seem to be influenced by temperature, make the bulk Mn/Fe ratio in lavas a good lithological tracer, supporting previous claims. Hence, we show that natural compositions can be used to expand trends in FRTE distribution behavior across a wider range of temperatures (500-1500∘C) and compositions than determined previously by experiments alone.

Poster presentation: 16 December
Abstract: V038-014
Session V038: Intraplate Volcanism and Deep Volatile Cycling: Insights into the Dynamic Processes in the Solid Earth
The quantification of mantle heterogeneity and its contributions to magma genesis are crucial for our understanding of the nature of Earth’s geochemical reservoirs and recycling processes. Today, the source of basalts is often envisioned as a heterogeneous mantle that comprises a range of lithological heterogeneities, especially pyroxenites, introduced into the mantle by various geodynamic and magmatic processes. Different chemical parameters have been proposed to trace evidence for the contribution of lithological heterogeneities during magma genesis (e.g., major elements [1-2], major element ratios [1,3] or logratios [4], first-row transition element concentrations [5-6], iron isotopes [7] and several empirical models for partial melting of a heterogeneous mantle have been developed in an attempt to quantify the proportion of pyroxenites in the mantle source of magmas [8-11]. However, the large range of compositions covered by the potential lithologies present in the mantle makes it challenging to determine a unique proxy for their contributions in the magmas [2,12]. Additionally, these contributions are controlled by various factors, such as the abundances of the different lithologies in the mantle, their respective melting behaviors (solidus temperatures and melt productivities) and the thermal and melting regimes of the mantle. Finally, interpretations from the magma compositions are complicated by the potential presence of volatiles in the mantle source and/or by modifications experienced by the magmas during their journey through the mantle [8,12]. This keynote presentation will present examples of challenges that can rise when we try to quantify the nature and abundance of the mantle heterogeneity, the efforts that have been published by various authors, and a few potential research directions that could bring prospects for success.
[1] Hauri (1996), Nature 382, [2] Lambart et al. (2013), Lithos 160-161, [3] Hirschmann et al. (2003), Geology 31, [4] Yang et al. (2019), JGR-Solid Earth 124, [5] Sobolev et al (2007), Science 316, [6] Sobolev et al. (2008), Science 321, [7] Williams and Bizimis, EPSL 404, [8] Mallik and Dasgupta (2014), GGG 15, [9] Kimura and Kawabata (2015), GGG 16, [10] Lambart et al. (2016), JGR-Solid Earth 121, [11] Brown and Lesher (2016), GGG 17, [12] Mallik et al. (in press), in: Konter J., Ballmer M, Cottaar S, & Marquardt H. (Eds. ), AGU monograph.

Virtuam Presentation
Keynote Speaker
Q&A session: 23 June
1:30-2:30pm
Session 03a: Development and recycling of chemical and isotopic heterogeneities in the sub-arc mantle: observations, models and experiments
Goldschmidt, Virtual
21-26 June 2020
Natural forsterite(Fo)-rich olivines represent the major constituent of the Earth's upper mantle. Collected at the surface in mantle xenoliths, they are commonly used in scientific research. Many electron microprobe labs use the San Carlos standard USNM111312/44 [1], provided by the Smithonian Institution, for calibrations. Non-USNMdistributed crystals of San Carlos olivine are also often used as starting material in experimental studies [e.g., 2, 3]. However, the potential inherent chemical variability of starting materials can affect results and their scientific interpretations. Hence, it is important to characterize the full chemical variability of the San Carlos olivine. Fournelle [4] showed that the USNM material shows limited variability (Fo89.6 to Fo90.5), but that non-standard San Carlos olivines can be significantly more variable, with Fo contents ranging from 87 to 92%. Following these results, we report new major and trace element analyses on grains (0.5 mm - >5mm) of non-USNM San Carlos olivine and compare them with analyses on USNM San Carlos standard compositions. We also investigate the presence of potential grain-scale chemical variations by looking at composition profiles on large (> 5mm) grains. Observed major-element variations (Fo88.4 to Fo91.4) are consistent with Fournelle’s results [4]. Additionally, we show that minor and trace element concentrations present significant and contrasted variations between grains (e.g., 17 % Ni, 28 % Mn, 44% V, 69 % Al, 285 % P, relative). At the scale of the individual grain, however, San Carlos olivines appear relatively homogeneous with no systematic core-rim variations. Results and implications for the use of this material in experimental studies and for interpretations of the petrogenetic processes will be discussed.
[1] Jarosewich et al. (1979), Smithsonian contrib. to the earth sciences 22. [2] Demouchy & Mackwell (2006), Phys Chem Minerals 33. [3] Wang & Gaetani (2008), CMP 156. [4] Fournelle (2011), Microsc. Microanal. 17.

Virtuam Presentation
Q&A session: 24 June
1:30-2:30pm
Session 03g: Earth’s lithosphere formation and evolution from Hadean to Modern
Goldschmidt, Virtual
21-26 June 2020
Mid-ocean ridges provide an excellent environment in which to study the evolution of magmatic systems. Geophysical data indicate that lower crustal magma reservoirs comprise crystal-rich and magma-rich portions, but their properties remain uncertain. Image analysis of glomerocrysts derived from crystal-rich parts of the magma plumbing system indicates that they contain a range of melt proportions, averaging ~5% [1]. Melt inclusions hosted in plagioclase antecrysts indicates that these mushy magma reservoirs may be vertically extensive, extending to >16 km depth [2].
Within crystal-rich portions of the magma plumbing system, a number of processes operate which exert first-order controls on the compositions of extracted lavas. The first is reactive porous flow [3]. A combination of new data and experiments indicate that reactions between crystals and melts are very efficient, and that they operate through a dissolution-reprecipitation mechanism [4]. The second process is mixing. Recent data show that clinopyroxene and plagioclase contain nearly the full Nd isotopic heterogeneity observed along the northern Mid-Atlantic Ridge, including hotspots such as Iceland and the Azores, even though the associated lavas are isotopically homogeneous [5]. These data require efficient mixing.
Efficient melt extraction from the lower crust is required to form mid-ocean ridge basalt, but this process is poorly understood. Data for fast-spreading lower crust suggest that extracted melts are in equilibrium with the deeper portion of the lower crust, but not with the plutonics that formed in the melt-rich body located at the base of the sheeted dykes [6]. This indicates that melt extraction from the lower crust occurs by episodic channeled flow that is rapid enough to prevent significant mixing or reequilibration with high-level plutonic rocks. Together with the evidence for reactive flow, this suggests that melt transport in mid-ocean ridge magma reservoirs occurs both by continuous low-porosity porous flow and episodic, high-porosity focused flow.
[1] Bennett EN (2019) Ph.D. thesis, Cardiff University. [2] Bennett EN et al. (2019) Nature 572:235-239. [3] Lissenberg, CJ and MacLeod CJ (2016), J Pet 57:2195-2220. [4] Yang AY et al. (2019), Geochemistry, Geophysics, Geosystems. [5] Lambart S et al. (2019), Nat Geosci 12:482-486. [6] Loocke MP (2016) Ph.D. thesis, Cardiff University

Keynote: 8 January
13:45-14:15
Mariners Suite - Crowne Plaza hotel
Session 1 - Processes at Plate Margin
VMSG, Plymouth, UK.
7-9 January 2020
Most of what we know about the mantle heterogeneity comes from secondary sources of information. For example, mid-ocean ridge basalts (MORBs) have been the primary tool to map the geochemical heterogeneity of the oceanic upper mantle for decades [1-3]. However, MORB homogenize via magma mixing prior to eruption and, hence, may not capture the full chemical variability of their mantle source [4-5].
In this study [6], we performed high spatial resolution Sr-Nd isotope analyses on clinopyroxene and plagioclase from lower crustal gabbros drilled on a depleted ridge segment of the northern Mid-Atlantic Ridge (i.e., the Atlantis Massif) and compared the isotopic heterogeneity of melts delivered to the lower crust with those erupted onto the seafloor.
We find that (1) isotopic heterogeneity in the primitive cumulates occurs down to the centimeter-scale, with plagioclase and clinopyroxene from individual samples commonly not in equilibrium; (2) the cumulates are significantly more heterogeneous than the associated diabase and lavas and contain nearly the full Nd isotopic heterogeneity of all of North Atlantic MORB (including hotspots).
Our data require that magma mixing during melt transport and extraction from the mantle is limited, and that the isotopic homogeneity of MORB on a regional scale is instead the result of extensive mixing at shallower pressure in the crust. Furthermore, modelling indicates that North Atlantic data can be reproduced by melting a depleted peridotite source containing 3.2% to 8.5% of enriched recycled crustal material and a restricted range of potential temperatures (1245°C-1367°C).
[1] Hart, S. R. (1988). Earth Planet. Sci. Lett. 90, 273–296; [2] White, W. M. (1985). Geology 13, 115–118; [3] Cohen, R. S. et al. (1980). Nature 283, 149–153; [4] Batiza, R. (1984). Nature 309, 440–441; [5] Cipriani, A. et al. (2004). Geology 32, 657–660; [6] Lambart et al. (2019). Nature Geoscience, doi: 10.1038/s41561-019-0368-9

INVITED
Oral Presentation: 9 December
16:45-17:00
Moscone South - 154, Upper Mezz.
Session V14C - The Structure, Composition, and Dynamics of Earth’s Mantle and Crust Through Intraplate and Mid-Ocean Ridge Volcanism II
AGU FM 2019, San Francisco, CA.
9-13 December 2018
Radiogenic isotope signatures of mid-ocean ridge basalts (MORB) have been used for decades to map mantle composition, defining the depleted mantle end member. However, MORB homogenize via magma mixing prior to eruption and, hence, may not capture the full chemical variability of their mantle source. Here we show that substantial mantle heterogenity is preserved in the lower crust, providing strong evidence that the limited isotopic variability recorded in MORB is a consequence of crustallevel mixing of melts from a highly heterogeneous source. We performed high spatial resolution Sr-Nd isotope analyses on clinopyroxene and plagioclase from lower crustal gabbros drilled on a depleted ridge segment of the northern MidAtlantic Ridge. These primitive cumulate minerals record nearly the full heterogeneity observed along the northern Mid-Atlantic Ridge, including hotspots. Furthermore, we find that isotopic heterogeneity occurs down to the sample scale, with plagioclase and clinopyroxene from individual samples commonly not in isotopic equilibrium.
We demonstrate that MORB and cumulate mineral data can be reconciled with high magnitude, small length scale heterogeneity throughout the North Atlantic upper mantle. Modelling indicates that North Atlantic data can be reproduced by melting a depleted peridotite source containing 3.2% to 8.5% of enriched recycled crustal material and a restricted range of potential temperatures (1245°C-1367°C). Furthermore, the data require that magma mixing during melt
transport and extraction from the mantle is limited, and that the isotopic homogeneity of MORB on a regional scale is instead the result of extensive mixing at shallower pressure in the crust.
Oral Presentation: 23 August
14:30am-14:45am
Room 212
Session 02c: Evolution of the silicate earth: celebrating Al Hofmann’s contributions to Geochemistry
Goldschmidt, Barcelona
18-23 August 2019
Major-, trace elements and isotopic compositions reveal the presence of recycled heterogeneities in the sources of Ocean Island Basalts (OIBs). However, the lithology, compositions and proportions in the upper mantle are debated. The high Mg# in primary OIBs require contributions of peridotite along with recycled non-peridotitic heterogeneites [1,2]. Here we estimate the proportion of recycled heterogeneites in the sources of primary OIBs globally, to illustrate both the importance of the melting model and the role of the lithology and composition of the heterogeneites.
We constrain the proportion of heterogeneity in the OIB sources for two distinct pyroxenite compositions, using two melting models for primary OIB formation – (a) simple mixing model between pyroxenite- and peridodite-derived melts [3]; (b) a reactive crystallization model of the pyroxenite-derived melt during its ascent through a peridotitc mantle [4]. We use the parameterizations of [3], [4], [5] and [6] in our calculations.
We show that (a) proportions of constrained heterogeneity under the same ocean island are very dependent on the melting model and composition of heterogeneity; (b) irrespecive of the choice of model or composition, a significantly heterogeneous source is found beneath all ocean islands; (c) proportion of heterogeneity in the source has no correlation with mantle potential temperature or final pressure of melting, implying a decoupling between the thermal state and chemical heterogeneity in the upper mantle. We discuss how such a decoupling has implications for why OIBs display greater heterogeneity in major element compositions than mid-ocean ridge basalts.
[1] Sobolev et al. (2005) Nature 434, 590–597. [2] Mallik & Dasgupta (2012) EPSL 329–330, 97–108. [3] Lambart et al. (2016) JGR 121, 5708–5735 [4] Mallik & Dasgupta (2014) G3 15, 1533–1557. [5] Sobolev et al. (2007) Science 316, 412–417. [6] Herzberg & Asimow (2015) G3 16, 563–578.
Oral Presentation: 21 August
10:15am-10:30am
Room 131+132
Session 06a: Mantle2Crust: Basalt Genesis, Transport and Differentiation
Goldschmidt, Barcelona
18-23 August 2019
The Atlantis Massif provides a unique opportunity to study crustal accretion at a slow-spreading ridge. The massif is a large undersea dome shaped region located along the Mid-Atlantic Ridge within the Atlantic Ocean. Tectonics involved in the formation of the massif 1.5 to 2 million years ago are due to low angle detachment faulting. The eastern portion of the massif represents volcanics of the hanging wall, while the central and southern portions represent the footwall containing intrusives characteristic of an ultramafic oceanic core complex. In 2004 and 2005, the Integrated Ocean Drilling Program (IODP) carried out expeditions 304 and 305 to extract core samples from the Atlantis Massif. These rocks are composed of gabbros and are useful in mid-ocean ridge basalt (MORB) research. MORB’s are used as a tool to gain a better understanding of the upper mantle and crustal processes. Most of prior assumptions to explain melt evolution in oceanic crustal magma chambers relied solely on fractional crystallization. However, MORB are likely to undergo many other modifications prior to eruption, such as magma-mixing or melt-rock reaction. In order to interpret MORB composition correctly, we need to understand and quantify the effects of these processes, which is the focus of this research project. The objective is to assess MORB evolution and crystallization history of the lower crust by investigating these crustal gabbroic rocks, and to ultimately compare results with a separate spreading oceanic ridge within the East Pacific Rise. This can be done by performing major and trace elemental analysis through electron microprobe analysis (EMPA) and Laser Ablation-Inductively Coupled Plasma- Mass Spectrometry (LA-ICP-MS); respectively. Data from both analyses are then combined into a Magnesium and Rare Earth Element (Mg-REE) coupled geo-speedometer, which will place pressure and temperature constraints along the Atlantis Massif to determine a defined rate of crustal expansion.

Poster: 11 August
14:15pm-15:15m
Student Recreation and Activity Center - Tripod 41 Side B
Session: Poster3
National Conferences on Undergraduate Research,
Kennesaw State University, GA
11-13 April 2019
Variations in radiogenic isotopes in mid-ocean ridge basalts (MORB) are interpreted to reflect the presence of enriched and depleted mantle components in their source regions and have been used to infer the abundance and time scales of crustal recycling. However, MORB are homogenized via magma mixing prior to eruption and may not capture the full heterogeneity of melts generated in their upper mantle source. Here we show that primitive cumulate minerals, formed by crystallization of mantle melts in the lower crust, retain the signature of the recycled material. We performed high spatial resolution Nd and Sr isotopic analyses on clinopyroxene and plagioclase of gabbroic cumulates from the Atlantis massif, located on a depleted ridge segment on the northern Mid-Atlantic Ridge, and compared these data with whole rock isotopic compositions of diabase and microgabbros collected on the same core, associated basalts flows, and MORB data from the literature.
We find that cumulate minerals: (1) are significantly more isotopically heterogeneous than the associated diabase and lavas, exceeding the range of 143Nd/144Nd in MORB by a factor of seven; and (2) contain the full Nd isotopic heterogeneity of all of North Atlantic MORB. Furthermore, we find that isotopic heterogeneity occurs down to the sample scale, with plagioclase and clinopyroxene from individual samples commonly not in isotopic equilibrium. We further demonstrate that the MORB and cumulate mineral data can be reconciled with constant high magnitude, small length scale heterogeneity through the North Atlantic upper mantle, with limited magma mixing in the mantle and extensive mixing in the oceanic crust.
The isotopic heterogeneity revealed in the lower oceanic crust provides strong evidence that MORB is not an accurate representation of the heterogeneity of its mantle source. Hence, the true isotopic variation of the upper mantle requires rigorous further examination, and models of convective thinning and stretching and melt migration must be re-evaluated to account for greater local variation.
Plain-Language Summary:
The rocks that make up Earth's oceanic crust are subject to a life cycle - new oceanic crust is formed at divergent plate boundaries (mid-ocean ridges) via the melting of the mantle (material beneath the crust) and transport of magma to the surface, while old crust sinks back into the mantle at convergent plate boundaries (subduction zones). The return of oceanic crust to the mantle creates chemical and mineralogical heterogeneities. Lavas produced at mid-ocean ridges have been used for the last five decades to map these heterogeneities and inform our understanding of Earth's geodynamics. Here we show that these lavas do not provide an accurate representation of the upper mantle heterogeneity. Rather, minerals in plutonic rocks, forming the lower part of the oceanic crust, reveal that the mantle is significantly more heterogeneous than previously inferred from lavas.

Oral Presentation: 11 December
14:40pm-14:45pm
Marriott Marquis - Liberty M
Session V23A: A Multidisciplinary Approach to Investigating the Formation of Earth and Its Chemically Distinct Reservoirs I
AGU FM 2018, Washington, D.C.
10-14 December 2018
Melting in a lithologically heterogeneous mantle is expected to impact magma supply, crustal thickness, and magma transport. Oceanic basalts are products of mantle melting and thus potential indicators of such heterogeneity, but basalts record complex histories that can be difficult to uniquely interpret. Uranium-series isotopes are well-suited to fingerprinting diverse lithologies due to variations in 1) source mineralogy and U-Th-Pa-Ra partitioning, and 2) lithologic melt fertility and the series’ particular sensitivity to melting rates. However, such interpretations to-date have been hampered by a lack of constraints on the melting behavior of mafic rocks under mantle conditions.
Recent, experimentally-based parameterizations for pyroxenite melting, and the application of those new calibrations to two-lithology melting calculations [Lambart et al., 2016, JGR; Lambart, 2017, GPL], constitute significant improvements to our predictions for pyroxenite melting. Here we use the outcomes of those parameterizations and of energy-constrained two-lithology melting calculations to produce a refined set of melt fractions and mineral modes during adiabatic melting. We tested the effects of melting upper mantle containing 10% pyroxenite for a range of mantle potential temperatures. In agreement with recent work [Lambart, 2017], we find that productivity variations for two-lithology melting regimes have a significant impact on resulting melt mixtures, with particular dependence on pyroxenite compositions and resulting solidus temperatures.
The resulting melt fractions and partition coefficients will next be used to determine U-series disequilibria for pyroxenite- and peridotite-derived partial melts and mixtures thereof. We will consider 1D, continuous numerical solutions for both dynamic melting and reactive porous flow scenarios, allowing both the degree of melting and the mineral/melt partition coefficients to vary non-linearly. We expect the resulting predicted basalt compositions to better approach the effects of heterogeneous mantle melting on U-series isotope disequilibria in basalts.
* Denote the speaker
Oral Presentation: 17 August
10:30am-10:45am
Room 311
Session 4i: Magma Genesis Beneath Oceans and Continents: Source Signatures and Melting Processes
Goldschmidt, Boston
12-17 August 2018
It is generally admitted that oceanic basalts are formed by continuous melting of the mantle [1]. However, most parameterizations are calibrated on batch melting experiments. Using pMELTS [2], I modelled the adiabatic decompression of a heterogeneous mantle (peridotite + pyroxenite) and compared the melt composition and productivity produced by both batch and continuous melting.
Calculations reveal two major flaws in using parameterizations based on batch melting rather than continuous melting:
(1) the proportion of solid pyroxenite in the mantle source might be strongly underestimated. Because most proposed mantle pyroxenite compositions are denser than peridotite [3], an increase in the pyroxenite proportion may affect the buoyancy of the mixture in the upper mantle. Applying the corrected proportion to Iceland, I show that maintaining a positive buoyancy of the mantle source requires either a potential temperature TP ≈ 1600°C notably higher than the recent estimates (i.e., 1450-1510°C [4,5]) or a significant proportion of less dense material, such as harzburgite (e.g., for TP = 1500°C, buoyancy is maintained if the mantle contained > 20% harzburgite).
(2) The Ni proxy used to constrain pyroxenite abundance in mantle source [6] might not be suitable for continuous melting regime. In fact, the Ni content of the integrated melt produced by continuous melting of a given lithology can be significantly lower than the one produced by batch melting, and Ni-rich, olivine-free pyroxenites might produce melts that are not enriched in nickel when compared with peridotite melts if continuous melting is considered.
References: [1] McKenzie (1984) J. Petrol 25; [2] Ghiorso et al. (2002), GGG 3; [3] Shorttle et al. (2014), EPSL 395; [4] Herzberg & Asimow (2015), GGG 16; [5] Matthews et al. (2016), GGG 17; [6] Sobolev et al. (2007), Science, 412-417.
INVITED
Oral Presentation: 13 August
03:00pm-03:15pm
Amphithéâtre havane
Session No. 05a: Morb Petrogenesis: From mantle partial melting to fractional crystallization
Goldschmidt, Paris
13-18 August 2017
Studying mineralogy is fundamental for understanding the composition and physical behavior of natural materials in terrestrial and extraterrestrial environments. However, some students struggle and ultimately get discouraged with course material because they lack well-developed spatial visualization skills that are needed to deal with three-dimensional (3D) objects, such as crystal forms or atomic-scale structures, typically represented in two-dimensional (2D) space. Fortunately, spatial visualization can improve with practice. Our presentation demonstrates a set of experiential learning activities designed to support the development and improvement of spatial visualization skills in mineralogy using commercially available magnetic building tiles, rods, and spheres. These instructional support activities guide students in the creation of 3D models that replicate macroscopic crystal forms and atomic-scale structures in a low-pressure learning environment and at low cost. Students physically manipulate square and triangularly shaped magnetic tiles to build 3D open and closed crystal forms (platonic solids, prisms, pyramids and pinacoids). Prismatic shapes with different closing forms are used to demonstrate the relationship between crystal faces and Miller Indices. Silica tetrahedra and octahedra are constructed out of magnetic rods (bonds) and spheres (oxygen atoms) to illustrate polymerization, connectivity, and the consequences for mineral formulae. In another activity, students practice the identification of symmetry elements and plane lattice types by laying magnetic rods and spheres over wallpaper patterns. The spatial visualization skills developed and improved through our experiential learning activities are critical to the study of mineralogy and many other geology sub-disciplines.
* Denote the speakers

Poster: 13 December
08:00am-12:20pm
Moscone South - Poster Hall
Abstract: ED21A-0760
ED21A: Activities and assignments that promote student learning in undergraduate earth and environmental science classes
AGU Fall meeting San Francisco
12-16 December 2016
Poster
Cracking caused by reaction-driven volume increase is an important process in many geological settings. In particular, the interaction of brittle rocks with reactive fluids can create fractures that modify the permeability and reactive surface area, leading to a large variety of feedbacks. The conditions controlling reaction-driven cracking are poorly understood, especially at geologically relevant confining pressures. We conducted two sets of experiments to study the effects of confining pressure on cracking during the formation of gypsum from anhydrite CaSO4 + 2H2O = CaSO4∙2H2O, and portlandite from calcium oxide CaO + H2O = Ca(OH)2. In the first set of experiments, we cold-pressed CaSO4, or CaO powder to form cylinders. Samples were confined in steel, and compressed with an axial load of 0.1 to 4 MPa. Water was allowed to infiltrate the initially unsaturated samples through the bottom face via capillary and Darcian flow across a micro-porous frit. The height of the sample was recorded during the experiment, and serves as a measure of volume change due to the hydration reaction. We also recorded acoustic emissions (AEs) using piezoelectric transducers (PZTs), to serve as a measure of cracking during an experiment. Experiments were stopped when the recorded volume change reached 80% - 100% of the stoichiometrically calculated volume change of the reaction. In a second set of experiments, we pressed CaSO4 powder to form cylinders 8.9 cm in length and 3.5 cm in diameter for testing in a tri-axial press with ports for fluid input and output, across the top and bottom faces of the sample. The tri-axial experiments were set up to investigate the reaction-driven cracking process for a range of confining pressures. Cracking during experiments was monitored using strain gauges and PZTs attached to the sample. We measured permeability during experiments by imposing a fluid pressure gradient across the sample. These experiments elucidate the role of cracking caused by crystallization pressure in many important hydration reactions.
* Denotes the speaker

Poster: 15 December
08:00am-12:20pm
Moscone South - Poster Hall
Abstract: MR41A-2680
Session: MR41A: Physical Properties of Earth Materials (PPEM): Rock Deformation over Various Time and Spatial Scales II
AGU Fall meeting San Francisco
12-16 December 2016
Thanks to numerous experimental studies, parameterizations are available to model the melting behavior of peridotite [1] and pyroxenite [2] compositions that are thought to be present in the mantle. Based on these parameterizations, numerous studies have attempted to estimate the proportion of pyroxenites in magmatic sources. However, while these parameterizations are mostly based on batch melting experiments, oceanic basalts are likely to be formed by near fractional melting rather than batch melting [3]. Using pMELTS [4], I investigated the effect of near-fractional melting of pyroxenite and applied the results to Iceland's basalt for which the proportion of pyroxenite-derived melt has been estimated to 20-30 % [5,6]. Calculations suggest that 30% of pyroxenite-derived melt in the aggregated magma can be generated by 5% pyroxenite in the source in a case of batch melting but highlight that at least 12% (i.e, more than twice the amount required with batch melting) pyroxenite in the source may be required for near-fractional melting. The buoyancy of a mantle with 5% of pyroxenite can simply be maintained with increasing the mantle potential temperature, TP, from 1300 to to 1440°C, but 12% of pyroxenite in the mantle required either TP ≈ 1600°C, i.e., a temperature significantly higher than the recent estimates (i.e., 1450-1510°C [7,8]) or a significant proportion of less dense material, such as harzburgite (e.g., for TP = 1500°C, buoyancy is maintained if the mantle contained > 20% harzburgite). Note these estimates are minimal as the plume buoyancy beneath Iceland is supposed to be strongly positive to meet the volume flux estimate [9].
References:
[1] Katz et al. (2003), GGG 4; [2] Lambart et al. (under review), JGR - Solid Earth; [3] Hirose & Kawamura (1994), Geophy. Res. Let 21, 2139-2142; [4] Ghiorso et al. (2002), GGG 3, 1030; [5] Sobolev et al. (2007), Science, 412-417; [6] Shorttle et al. (2014) EPSL 395, 24-40; [7] Courtier et al. (2007), EPSL 264, 308-316; [8] Herzberg & Asimow (2015), GGG 16, 563-578; [9] Jones et al. (2014), EPSL 386, 86-97.

Session 04g: Do basalts accurately sample mantle source rocks?
The role of lithologic heterogeneity in the sub-oceanic upper mantle during melt generation at ridges and hot spots
Goldschmidt, Yokohama
26 June - 01 July 2016
A variety of data requires that the mantle source for basaltic magmatism is heterogeneous. Thanks to numerous experimental studies, parameterizations are available to model the melting behavior of peridotite and pyroxenite compositions that are thought to be present in the mantle (e.g., 1, 2). Based on these parameterizations, numerous studies have attempted to estimate the proportion of pyroxenites in magmatic sources. However, while almost all melting experiments correspond to a batch melting process, it is likely that oceanic basalts are formed by near fractional melting rather than batch melting (e.g., 3). Due to the limited extent of melting of peridotites under upper mantle conditions, their magmatic productivity and melt compositions are similar for batch and fractional melting (e.g., 4). In contrast, pyroxenites undergo much higher meting degrees during decompression of a heterogeneous, peridotite-rich mantle source. Using pMELTS, I investigated the effect of near-fractional melting of pyroxenite. Results suggest that the nature of the melting process for pyroxenites can significantly affect (1) the melt productivity of pyroxenites and thus their potential contribution in basalt genesis, (2) the major element composition of melts and thus their interaction with the surrounding peridodite, and (3) the concentration of minor elements such as Ni and consequently the estimation of pyroxenite proportion in magma-source (e.g., 5). In particular, calculations imply that the proportion of solid pyroxenite in the magma source is likely to be underestimated using "batch melting" rather than "fractional melting" parameterization. An increase in the pyroxenite proportion may affect the buoyancy of the mixture in the upper mantle and have important geodynamical implications.
References:
1-Katz et al., 2003, GGG 4; 2-Lambart et al., 2013, Lithos 160-161; 3- Hirose & Kawamura, 1994, Geophy. Res. Let 21; 4-Johnson et al., 1990, J. Geophy. Res. 95; 5-Sobolev et al., 2007, Science 316

Poster: Wed. December 16th
08:00am-12:20pm
Paper No. DI31B-2582
Session: DI31B Melt and Liquids in Earth and Planetary Interiors
AGU Fall Meeting, San Francisco
14-18 December 2015
It is generally believed that melt-peridotite interaction in the upper mantle plays an important role in melt transport. A major aspect of these interactions is the coupled dissolution of pyroxene and precipitation of olivine, or vice versa. Pyroxene dissolution and olivine precipitation increase the proportion of melt in the system and facilitate its transport by increasing the porosity and permeability of mantle rocks [1,2]. This process has also been suggested for the extraction of pyroxenite-derived melt with little interaction with the peridotite mantle [3,4]. Natural occurrences of this reaction are found, for example, in the Krivaja-Konjuh massif in Bosnia [5], in mantle xenoliths from French Polynesia [6], and in the Balmuccia massif in Italy [7]. Inversely, pyroxene crystallization at the expense of olivine would lead to a porosity reduction and increase the lithological variability of the mantle [8,9]. However, melt-peridotite interaction processes depend on many chemical and physical parameters such as the composition of the melts, and the nature of the transport mechanism (pervasive porous flow, focused flow or magma transport in dikes), and are very difficult to model in the laboratory.
First I will review the experimental and theoretical efforts that have been published to constrain the effect of melt-rock interaction on the lithological variability of the mantle as functions of the melt composition, the melt-rock ratio and the pressure-temperature conditions. Then I will discuss the conditions to reproduce the rock suite observed in the Krivaja-Konjuh massif and how they differ from the conditions in the Balmuccia massif and the French Polynesia xenoliths.
References:
[1] Kelemen et al., 1990, J. Petrol. 31(1), 51-98; [2] Daines & Kohlstedt, 1994, GGG 21(2), 145-148; [3] Lundstrom et al., 2000, Chem. Geol. 162, 105-126; [4] Hirschmann et al., 2003, Geology 31(6), 481-484; [5] Faul et al., 2014, Lithos 202-203, 283-299; [6] Tommasi et al., 2004, EPSL 227, 539-556; [7] Mazzucchelli et al., 2009, J. Petrol 50(7), 1205-1233; [8] Lambart et al., 2012, J. Petrol. 53(3), 451-476; [9] Sobolev et al., 2005, Nature 434, 590-597.
Geological Society of America Abstracts with Programs. Vol. 47, No. 7

INVITED
Oral Presentation: 3 November
11:30am-11:45am
Room 331/332
Paper No. 195-13
Session No. 195:
T162. A Lower Crustal Perspective on Magmatic Arc Processes
GSA Annual Meeting
Baltimore
1-4 November 2015
Carbonation of peridotite may be an important sink in the global carbon cycle. Some natural systems attain 100% carbonation. In these systems, all Mg, Ca and Fe is extracted from silicate minerals to form carbonate minerals, perhaps as a result of reaction-driven cracking processes that maintain or enhance permeability and reactive surface area. Without reaction-driven cracking, in situ mineral carbonation could fill pore space and armor reactive surfaces, limiting reaction progress and leaving much of the silicate unaltered (Kelemen and Hirth, 2012).
The reactive-cracking process will occur if stress resulting from anisotropic volume changes during fluid-rock interactions, i.e., the "crystallization pressure", is sufficient to fracture rocks. The crystallization pressure is usually described as:
P' = -ΔGr/ΔVs (1)
where P' is the crystallization pressure, ΔGr is the Gibbs Free Energy of a reaction, and ΔVs is the change in solid volume resulting from this reaction (Scherer, 2004; Steiger, 2005). Kelemen and Hirth (2012) proposed that use of Helmholtz Free Energy of reaction ΔFr may be preferable, yielding:
P' = -ΔFr/ΔVs = -ΔGr/ΔVs + PΔVr/ΔVs (2)
where P is the confining pressure and ΔVr is the volume change of a stoichiometric reaction including fluid components.However, the conditions most favorable for reaction-driven cracking are poorly understood, especially at confining pressures relevant to CO2 storage. Fundamental uncertainties remain in estimating the crystallization pressure that drives reaction-driven cracking and, so far, experiments on peridotite hydration or carbonation have not produced reactive cracking, possibly due to limited reactive surface area in low porosity samples. To address this, we explore crystallization pressure in simple systems under controlled laboratory conditions.
We have performed experiments on reaction (1): solid CaO + H2O = solid Ca(OH)2, with a 100% increase in the solid volume and high initial porosities (Φ). Using equation (1), the calculated pressure of crystallization of Ca(OH)2 is 4.1 GPa. However, equations (1) and (2) do not incorporate energy sinks such as exothermic heating and/or thermal diffusion, implicitly assuming that all chemical potential energy is converted into stress. Thus, both equations yield an upper bound for P'.
We cold-pressed CaO powder to form ∼20 mm long cylinders 4 to 12 mm in diameter, with initial Φ ranging from 0.36 to 0.53. These cylinders were confined in steel, and compressed with an axial load of 0.1 to 27 MPa while water was introduced through a micro-porous frit. Without expansion of the total volume, the reaction would stop when Φ = 0, producing Φ = 0, 2∙Φ∙VCa(OH)2+ (1-2∙Φ)∙VCaO.
Instead, in all experiments the volume of cylinders increased with time, maintaining Φ > 20%. Hence, the stress exerted by the crystallization of Ca(OH)2 is higher than the maximum axial load (27 MPa). 27 MPa is also higher than the tensile strengths of most of the rocks, suggesting that, without confined pressure, reactive-cracking can happen with this system. The evolution of ΔV is best fit by a power law. The reaction slows over time, either because of a reduction of permeability or consumption of the reactant. The fact that all experiments show the same slope (ΔV = btm with m ∼ constant) whatever if the porosity was decreasing or increasing during the reaction, supports the latter explanation. Hence, experiments were stopped at reaction extents from 42 to 100% but all would certainly reach 100% at longer durations.
To investigate the conditions leading to reaction-driven cracking in this system, we’ve begun a series of high pressure experiments in a triaxial deformation apparatus. We will map stress and strain resulting from CaO hydration at geologically relevant combinations of confining pressure, temperature, and volume change. The configuration that we chose is a CaO cylinder embedded in a relatively inert, porous, sandstone (i.e., mainly SiO2). CaO solid + H2Ofluid = solid Ca(OH)2 and CaO solid + CO2 fluid = solid CaCO3. Both have a big volume change. The configuration with has already been tested without confined pressure and the first macroscopic fractures appears after only 3min. Additional fractures continued to form during the next 15min, after what the sample fell apart.
References:
Kelemen PB, Hirth G, 2012, Reaction-driven cracking during retrograde metamorphism: Olivine hydration and carbonation: Earth Planet. Sci. Lett., v. 345-348, p. 81–89.
Scherer GW, 2004, Stress from crystallization of salt: Cement and Concrete Research, v. 34, p. 1613-1624.
Steiger M, 2005, Crystal growth in porous materials – I: The crystallization pressure of large crystals: J. Crystal Growth, v. 282, p. 455-469.
* Denotes the speaker

Keynote Presentation 3:
23 June - 8:30am-9:00am
Fifth International Conference on Accelerated Carbonation for Environmental and Material Engineering
New York City
21-24 June 2015
The presence of the Hawaiian plume is manifested by the Hawaiian swell [1] and voluminous eruption of Ni-rich lavas [2] with enriched isotopic compositions [3]. Here we estimate the conditions of melt generation needed to reproduce both features.
We used thermodynamic treatment for fractional melting [4] and melting parameterizations for pyroxenites [5] and peridotite [6] to determine pyroxenite contribution in magmas Xpx as functions of potential temperature TP, pyroxenite abundance in the source P, radius of the melting zone R and distance to the plume axis. The final pressure of melting is set to correspond with the base of the lithosphere (3 GPa) at the plume axis and increases with the distance from the axis [7]. The Hawaiian plume axis is thought to be currently between Loihi (L), Kilauea (K) and Mauna Loa (ML), which are 25 km, 32 km and 44 km radially away from the plume axis, respectively [3]. To determine Xpx, we assumed that magmas are accumulated melts produced on a circular sampling zone of 50 km diameter centered beneath each volcano [8].
Preliminary calculations show that for TP = 1525°C, P = 0.07 and R = 55 km, XpxML = 0.59, XpxK = 0.49 and XpxL = 0.45. XpxML and XpxK are similar to values suggested by [2]. Computed liquidus temperatures at 3 GPa are consistent with those of Hawaiian parental melts (1500-1520°C; [9]). XpxL is higher than suggested by [2] (XpxL = 0.09) but their estimate is based on only one glass analysis. Our model is also consistent with isotopic compositions: K and L have similar εNd, while ML is more enriched [3]. Finally, we can compute the density deficit using parameterization of [1] and relate it to the volume flux volume flux [10]: we obtain 3.2 km3/Yr, a value similar to the estimations based on the Hawaiian swell model [1].
References:
[1] Ribe & Christensen 1999, EPSL; [2] Sobolev et al. 2005, Nature; [3] DePaulo et al. 2001, GGG; [4] Phipps Morgan 2001, GGG; [5] Lambart et al. in prep, EPSL; [6] Katz et al. 2003 GGG; [7] Ito & Mahoney 2005, EPSL; [8] DePaulo & Stolper 1996, JGR; [9] Putirka 2008, Miner. Soc. Amer. Geochem. Soc.; [10] Turcotte & Schubert 2002, Cambridge Press.
Poster: 17 December
1:40pm-6:00pm
Abstract: V33C-4885
Session: The geochemical diversity of the mantle inferred from hotspots: Five decades of debate
AGU Fall Meeting
San Francisco
15-19 December 2014
Carbonation of peridotite may be an important sink in the global carbon cycle. In some natural systems, 100% carbonation of rocks is attained via reaction-driven cracking processes. Engineered systems that emulate such processes may provide relatively inexpensive CO2 capture and storage [1,2].
Volume changes during fluid-rock reaction lead to stresses in elastic host rocks, known as the "pressure of crystallization", that can cause fracture. Cracking can maintain or enhance permeability and reactive surface area in a positive feedback mechanism. So far, experiments on peridotite hydration or carbonation have not produced reactive cracking, possibly due to limited reactive surface area in low porosity samples. To address this, we've begun experiments on reaction (1): solid CaO + H2O = solid Ca(OH)2, with a 100% increase in the solid volume and high initial porosities (φ).
We cold-pressed CaO powder to form ~20 mm long cylinders 6 mm in diameter, with initial φ ranging from 0.36 to 0.53. These cylinders were confined in steel, and compressed with an axial load of 0.1 to 4.2 MPa while water was introduced through a micro-porous frit. Without expansion of the total volume, the reaction would stop when φ = 0, producing 2⋅φ⋅Ca(OH)2 + (1-2⋅φ)⋅CaO.
Instead, in all experiments the volume of cylinders increased with time, maintaining φ > 20%. Experiments were stopped at reaction extents from 82 to 100% and would probably reach 100% at longer durations. The pressure of crystallization for reaction (1) is then > 4.2 MPa. Experiments performed on boreholes containing demolition mortar, largely CaO, demonstrate that this pressure is sufficient to break rocks [3].
Reaction (1) is rapid, which allowed us to perform numerous experiments but at higher axial loads Ca(OH)2 may flow viscously. In the future we plan similar experiments on ground, cold pressed olivine + H2O + CO2. At elevated temperatures, reaction progress in unconfined powders is > 80% in a few hours [4].
References:
[1] Kelemen and Hirth 2012 EPSL; [2] Kelemen et al. 2011 AREPS; [3] Kelemen et al. 2013 AGUFM; [4] Gadikota et al. 2014 Phys Chem Chem Phys.
* Denotes the speaker
Poster: 16 December 2014
Abstract: V23A-4768
Session: Achieving negative carbon emissions: Distributed carbon capture from air and surface water using geologic materials and/or storage reservoirs
AGU Fall Meeting
San Francisco
15-19 December 2014
Melting of mantle sources with multiple rock types, each with their own melting behavior and chemical and isotopic properties, is believed to be an important factor in producing the range of magma types characteristic of individual igneous provinces. An important example of such a compound source is a peridotitic mantle with minor pyroxenitic veins. Melting models of such mixtures require knowledge of the relationships between melt fraction (F), temperature (T), pressure (P), and bulk composition (X) for both peridotites and pyroxenites. While various parameterizations are available to model the melting behavior of peridotites, none yet exist to model pyroxenite melting.
Although empirical melting parameterizations are a useful and simple way to incorporate melting into tectonic models and have the advantage that they often better reproduce existing experimental data than exclusively thermodynamically models, current experimental data for pyroxenite melting are too sparse and show a large amount of scatter. Hence, we took an approach that incorporates thermodynamical concepts, using pMELTS [1] to guide our choice of functional forms, and calibrates them against the current experimental database. In this way, our parameterization becomes a reliable tool for compositions not used to calibrate the model.
Our model, PX-MELT is calibrated on 193 experiments (20 bulk compositions) in which F has been determined up to the cpx-out for pyroxenites over a substantial range of P and T (0.9-5 GPa; 1150-1675°C) and the parameterization is of the form of:
F = A·T′2 + B·T′ + 5 with T′=(T - T5%)/(Tcpx - T5%)
where F is the weight fraction of melt present, T5% is the temperature at F = 5%, Tcpx is the temperature of clinopyroxene exhaustion (cpx-out) in the pyroxenite assemblage and A and B are functions of the pressure (P, in GPa) and of the bulk composition of the pyroxenite.
PX-MELT, reproduces the melting degree undergone by a pyroxenite with a mean standard deviation of 11% absolute, T5% and Tcpx are reproduced with a standard deviation of 30°C and 32°C over a temperature range of ∼ 500°C, respectively. To measure the success of our melting parameterization, we used the variance reduction [2], i.e. the percentage of the total variance of experimentally determined F explained by the parameterization. The mean variance reduction on the 18 compositions used in the model is 83%. This value is similar to those obtained with parameterizations on peridotite melting :(72-85%) and is much higher than the value obtained with pMELTS for this experimental dataset (55%).
While remaining mathematically simple, PX-MELT succeeds in capturing the important features of the behavior of pyroxenites melting. It can be used to model the partial melting of multilithologic mantle sources, including the effects of varying the composition and the modal proportion of pyroxenite in such source regions. Several examples will be presented and the potential implications for the magma production and transport will be discussed.
References:
[1] Ghiorso et al. 2002, GGG; [2] Katz et al. 2003 GGG

Oral presentation: 8 May
Session: Subcontinental mantle lithosphere / melt-rock reactions
Sixth International Orogenic Lherzolite Conference
Marrakech, Morocco
4-15 May 2014
As the primary flux of material from the mantle to the surface, the basalts erupted at mid-ocean ridges (MORB) are a key resource for investigating the mantle's chemical composition. However, despite the large volumes of oceanic lithosphere returned to the mantle by subduction, it has proven difficult using basalt chemistry alone to quantify this material's involvement in melt production. Even more enigmatic is the signal of refractory material in the source, which may barely melt if other more fusible lithologies are present and so be difficult to identify from many common chemical tracers. Here we demonstrate how combining thermodynamic models of melting, the density of phase assemblages at high pressure and geochemical observations, can allow the proportion of refractory and enriched material in the mantle source to be estimated and place limits on mantle potential temperature.
We focus on determining the abundance of recycled material in the mantle beneath Iceland, where we have excellent geophysical and geochemical constraints on the melting process and the chemical variability in the mantle. Firstly, the lithologies contributing to melting are identified by quantitative comparison of the major element composition of erupted basalts to a database of experimental partial melts (Shorttle and Maclennan, 2011). Secondly, a mass balance is calculated between the endmember basalt compositions and the fully mixed melt to obtain the relative proportion, by mass, of enriched and depleted melts. A three-lithology melting model is then developed (peridotite-harzburgite-basalt), which uses the appropriate melting parametrisations to account for the differences in productivity between each lithology. The melting model enables the calculated abundance of the different endmember melt compositions to be projected back into mass fractions of solid mantle domains.
Applying this method to Iceland demonstrates that ~10% of the source is recycled basaltic material and at least 20% must be highly refractory and essentially un-melting. Combining geophysical constraints with the modelled high pressure densities of the three lithologies' assemblages constrains excess mantle temperature beneath Iceland to be at least 150°C. We extend the crustal thickness and geochemical constraints south along the Reykjanes ridge to show that Iceland represents a long wavelength lithological and thermal anomaly in the mantle - and that both lithology and temperature must be varying along ridge to match observations. Density modelling shows that the proportion of recycled basaltic material carried in the Iceland plume is near the limit of what maintains plume buoyancy in the shallow mantle.
References:
References: Shorttle O. and Maclennan J. Compositional trends of Icelandic basalts: Implications for short-lengthscale lithological heterogeneity in mantle plumes. Geochemistry, Geophysics and Geosystems, 12(11):Q11008, 2011.

Poster: 10 December 2013
Abstract: DI21A-2246
Session: Linking the Earth's Surface With the Deep Interior: Comparing Predictions and Observations of Mantle Plumes II
AGU Fall Meeting
San Francisco
9-13 December 2013
As the primary flux of material from the mantle to the surface, the basalts erupted at mid-ocean ridges (MORB) are a key resource for investigating the mantle's chemical composition. However, despite the large volumes of oceanic crust returned to the mantle by subduction, it has proven difficult to estimate the abundance of this recycled material in the mantle source using the chemistry of MORB. This is a significant problem, as fundamental questions about the dynamics of our planet cannot be answered without quantifying the abundance and spatial distribution of the mantle's chemical heterogeneity: is Earth's convection layered, or does it involve the whole mantle? What is the eventual fate of recycled oceanic material? What are the fluxes of elements from the deep Earth to the surface? Here, we present a method to estimate the proportion of enriched material in mantle source regions by combining geochemical observations with simple bilithologic models of mantle melting.
Working backwards from the chemistry of an erupted basalt to the proportions of peridotite and basalt lithologies in the source requires a number of critical pieces of information. Firstly, the geochemical variability at a ridge segment needs to be used to identify the types of lithology melting. Secondly, the relative mass of the enriched and depleted melts needs to be determined. Finally, a bi-lithological melting model needs to be run in an attempt to account for the over-representation of fusible, productive lithologies in the final mixed melt (Hirschmann and Stolper, 1996; Shorttle and Maclennan, 2011). This last step involves a large number of secondary assumptions/inputs to make the melting problem tractable, such as the mantle flow field and mantle potential temperature.
We determine the abundance of recycled material in the mantle beneath Iceland and at other ridges and ocean island settings in three stages. (1) The lithologies contributing to melting are identified by quantitative comparison of the major element composition of erupted basalts to a database of experimental partial melts (Shorttle and Maclennan, 2011). (2) A mass balance is calculated between the endmember basalt compositions and the fully mixed melt to obtain the relative proportion of enriched and depleted melts. (3) The bilithologic melting model from Shorttle and Maclennan (2011) is then used with the appropriate lithological melting parametrisations to account for the differences in productivity.
Applying this method to Iceland demonstrates that ∼ 10 % of the source is recycled basaltic material. However there are large uncertainties on this number, and our results demonstrate that the ability to constrain the mass fraction of lithologies contributing to melting depends heavily on the dynamics of mantle flow, melting and melt transport/reaction.
References:
Hirschmann M.M., Stolper E.M. A possible role for garnet pyroxenite in the origin of the garnet signature in MORB. Contributions to Mineralogy and Petrology, 124:185-208, 1996.
Shorttle O. and Maclennan J. Compositional trends of Icelandic basalts: Implications for short-lengthscale lithological heterogeneity in mantle plumes. Geochemistry, Geophysics and Geosystems, 12(11):Q11008, 2011.
Poster: 11 April 2013
Abstract: EGU2013-8312-2
Session: Materials, evolution and dynamics of the mantle
European Geosciences Union, General Assembly
Vienna, Austria
7-12 April 2013
Geochemical and isotopic data suggest that the source regions of MORBs and OIBs may be mixtures of peridotite and pyroxenite [1]. Models of the melting of such mixtures require knowledge of the relationships between melt fraction (F; expressed as percent), temperature (T), pressure (T), and bulk composition (X) for both peridotites and pyroxenites. While various parameterizations are available to model the melting behavior of peridotites [2,3], none yet exist to model pyroxenite melting. We have used 243 high-P experiments (22 bulk compositions) in which F has been determined up to the disappearance of clinopyroxene (i.e., cpx-out) for pyroxenites over a substantial range of P and T (0.9-5 GPa; 1150-1675°C) to constrain a model that can be used to estimate T from near the solidus (i.e., T5% the T at which F = 5%) up to cpx-out (Tcpx) for mantle pyroxenites spanning a wide compositional range extending to fertile peridotites.
Following [4], we assume that F is a monotonic, quadratic function of T from the solidus up to cpx-out. We first fit F as a function of T for each set of experiments at constant P on a given bulk composition; using these fits of F vs T, we then estimated T5% and Tcpx for each bulk composition. Because the uncertainty on the calculated T (T5% or Tcpx) increases with the difference between this T and the nearest experiment (in T-F space), fits where this distance is >50°C have not been used. Four studies on pyroxenites where Tcpx was closely bracketed but F not determined were also included in the database, yielding a total of 42 "T5% values" and 56 "Tcpx values".
Guided by pMELTS calculations [5], we have previously determined the functional form of T5% f(P,X) [6]. Based on previous studies [7], Tcpx was parameterized as a linear function of P and Xusing the results of high-pressure experiments from the database of [8]. We then used pMELTS to guide our modeling of the P and X dependences of the coefficients of the quadratic F vs T function between T5% and Tcpx. Tcpx =f(P,X). Although our modeling is not thermodynamic, our use of pMELTS, which is thermodynamically based, reduces the hazards of an arbitrary functional form.
When applied to the 243 experimental data, the model reproduces experimental F values with an average uncertainty of 15%, absolute; T5% of pyroxenites are reproduced with an average uncertainty of 18°C over a temperature range of ≈500°C. For fertile peridotite KLB-1 [9], our parameterization predicts T5% values of 1243, 1502, and 1689°C at 1, 3, and 5 GPa, respectively. For comparison, at the same pressures, the parameterization of [10] for fertile peridotite predicts solidus temperatures of 1248, 1473, and 1657°C.
In conjunction with estimates of heats of fusion for pyroxenite and peridotite lithologies, these parameterizations will permit calculations of how multilithologic mantle sources melt during adiabatic decompression, including the effects of varying the composition and the modal proportion of pyroxenite in such source regions.
References:
[1] Hofmann 1997, Nature;
[2] McKenzie and Bickle 1988, JPet;
[3] Katz et al. 2003 GGG;
[4] Pertermann & Hirschmann 2003, JGR;
[5] Ghiorso et al. 2002, GGG;
[6] Lambart et al. 2011, AGUFM #V32B-04;
[8] Putirka 2008, Rev.Miner. Geochem.;
[7] Hirschmann & al. 2008, GGG;
[9] Takahashi 1986, JGR;
[10] Hirschmann 2000, GGG.

Presentation: 7 December 2012
Abstract: DI51A-2343
Session: Mantle Plumes: What Do We Really Know?
AGU Fall Meeting
San Francisco
3-7 December 2012
Models of the contribution of mantle eclogites and pyroxenites to basalt petrogenesis depend on knowledge of the relationship between melt fraction (F), temperature (T), pressure (P), and bulk composition (X) for these lithologies and how this relationship compares to that of mantle peridotite. Here we present a parameterization of experimentally determined T at 5% melting for eclogites and pyroxenites as functions of P and X.
Using existing experimental data (14 bulk compositions, 162 experiments at 1-7 GPa and 1165-1750°C), we fit F as a function of T (at constant P) for each set of experiments on a given bulk composition and used these fits to estimate T at F = 5% melting (T5%). This exercise yielded a total of 32 "data points", each of which represents T5% for one of the 14 bulk compositions in the experimental data set at a given pressure. We limited our treatment to 5% melting since there are few experiments for any of the bulk compositions at F < 5%; moreover, at such low melt fractions, unknown factors such as dissolved water content in the melt can substantially influence the T vs F function.
We used pMELTS [1] to guide our choice of a functional form of T5% = f(P, X). The two most significant compositional parameters proved to be the bulk Na2O+K2O content [alk, in wt%] and the Mg# (= Mg/(Mg+Fe*), atomic, Fe* equals total Fe). The pMELTS calculations suggested the following functional form: T5% = P² (a·[alk] + b) + P (c·[alk] + d·Mg# + e) + F where a-f are fit parameters. When applied to the 32 experimental "data points", this expression reproduces the input T at F = 5% with an average uncertainty of 19°C over a T range of ~500°C; the maximum misfit is 41°C (see figure).
For the range of experimental bulk compositions used in our fit ([alk] = 0.57-3.71 wt%, Mg# = 0.590-0.898), the parameterization predicts T at F = 5% of 1124-1229°C at 1 GPa; 1251-1544°C at 3 GPa; and 1386-1967°C at 7 GPa. For comparison, the parameterization of [2] for a fertile peridotite predicts solidus temperatures (TS) of 1248, 1473, and 1799°C at 1, 3, and 7 GPa. Note that with increasing P, calculated TS values decrease relative to the range of T5% values.
Applying our parameterization to a global data set of mantle eclogites and pyroxenites suggests that for P of 4-7 GPa, approximately half of the compositions have T5% > peridotite TS at the equivalent P. The pyroxenites and eclogites with T5% < TS plot below the line: Mg# = 0.091·[alk] + 0.66, in [alk] vs Mg# space. As P decreases from 4 GPa, the percentage of compositions in the global data set with T5% < TS increases, reaching 100% at 1 GPa. Hence, our analysis suggests that not all olivine-poor heterogeneities in mantle sources can contribute substantial amounts of melt (i.e., have T5% < TS); the size of this contribution depends not only on the potential temperature of the upwelling mantle and the thickness of the overlying lithosphere, but also on the bulk composition of the pyroxenite/eclogite heterogeneities.
References:
[1] Ghiorso et al., 2002, GGG 3;
[2] Hirschmann, 2000, GGG 1
Geological Society of America Abstracts with Programs. Vol. 47, No. 7

Oral Presentation: 7 December 2011
Abstract: V32B-04
Video
Session: Mantle Melts: Innovative Approaches and Constraints to Modeling the Melting Regime II
AGU Fall Meeting
San Francisco
5-9 December 2011
Trace element and isotopic characteristic of alkali (i.e nepheline normative) basalts from ocean islands demonstrate that their source-region is heterogeneous [1]. However, the matter of lithologic or major element heterogeneity is still debated. A fundamental characteristic in terms of major elements of these basalts is their very wide variation in silica content for a given MgO content: the silica content ranges from less than 35 wt% to more than 50 wt%. To explain this variation, several assumptions have been suggested such as the variation of the melting rate of a peridotite ±CO2/H2O or various proportions of pyroxenites/eclogites in the source. However, these assumptions have a flaw: they do not take into account the compositional variability of mantle rocks. In fact, each "component" proposed as a possible source of OIB has been considered with a constant composition in terms of major element. Unlike peridotites, pyroxenites show a wide compositional range. Here we investigate if the compositional variability of mantle pyroxenites can explain the variability of alkalic oceanic basalts in terms of major elements.
We present an experimental study at 2 and 2.5 GPa on two natural pyroxenites from Beni Bousera: M5-40 is close to the mean of the natural pyroxenite population; M7-16 represents an iron-rich and silica-poor pole of this population. Solidi of M7-16 and M5-40 are located close to 1275°C and 1225°C at 2 GPa and close to 1325°C and 1375°C at 2.5 GPa, respectively. Melt compositions were analysed with the microdike technique [2] adapted for ½-inch assemblages. Pyroxenites present contrasted melting behaviours as a function of the phase proportion at the solidus. The high mode of garnet and the presence of olivine in the subsolidus assemblage of M7-16 yield to low-degree melts with very low silica content (36.7 - 41.0 wt%) and low aluminium content (9.3 - 13.2 wt%), very high FeO content (21.7 - 25.5 wt%) and high CaO/Al2O3 ratio (0.85 - 1.36). These major element features well correpond to HIMU-type basalts. Otherwise, the low-degree melts of M5-40 show typical EM1-type compositions according to Jackson and Dasgupta [3], characterized by high K2O contents (1.4 - 4.6 wt%), high K2O/TiO2 (0.7 - 3.8) and low CaO/Al2O3 (0.22 - 0.58) at moderate high SiO2 and FeO contents .
Thus we show that a large part of the major element variability of alkali oceanic basalt could be explained by the low degree partial melts of mantle pyroxenite if the compositional variability of the latter is taken into account. This has important geodynamical implications for the heterogeneous mantle. Solidi of pyroxenites are located at much lower temperature than the peridotite solidus at a given pressure and low-degree melts of pyroxenites for the origin of alkalic OIB implies strong constraints on mantle temperatures.
References:
[1] Hofmann (1997),
[2] Laporte et al. (2004),
[3] Jackson and Dasgupta (2008)

INVITED
Oral Presentation: 13 December 2010
Abstract: V13F-01
Session: Generation and Evolution of Alkaline to Subalkaline Magmas II
AGU Fall Meeting
San Francisco
13-17 December 2010
The involvement of pyroxenites has often been suggested to solve major problems in basalt petrogenesis (e.g., Hirschmann and Stolper, 1996, CMP, 124: 185-208). Numerous high pressure partial melting experiments of these rock-types allowed to better constrain their melting relations at pressures > 2 GPa. As the melting of pyroxenites can also contribute to basalt petrogenesis at pressures < 2 GPa, we performed partial melting experiments on three natural pyroxenites from Beni Bousera ultramafic massif (Morocco) at 1 and 1.5 GPa in a piston-cylinder apparatus: a garnet clinopyroxenite (M7-16), a garnet pyroxenite (M5-40), and a websterite (M5-103); M7-16 is silica-undersaturated, while M5-40 and M5-103 are silica-saturated. These three rocks cover the compositional cloud of pyroxenites worldwide, and can provide an insight into the links between bulk compositions and melting relations. Run products were characterized by scanning electron microscopy and electron probe microanalysis, and phase proportions were computed by mass balance. Our results suggest that bulk pyroxenite compositions control mineralogical assemblages, that in turn control solidus temperatures, melt productivities, and ultimately the relative contribution of pyroxenites and peridotites to basalt genesis. Melting intervals range from 110° C to 240° C and solidus temperatures range from 1160° C to 1220° C at 1 GPa and from 1200° C to 1265° C at 1.5 GPa; in the three compositions investigated, the solidus temperatures are lower and the productivities higher than those of dry peridotite. Depending on bulk composition and degree of melting, partial melting of pyroxenites at 1-1.5 GPa produces two kinds of liquids: (1) liquids with compositions close to those produced by peridotite melting, which may ascend to the surface without significant chemical exchanges with the surrounding peridotitic mantle; and (2) very silica-undersaturated and Fe-rich liquids, which are in strong disequilibrium with the peridotitic mantle and which may induce major changes of the composition of their host. Thus partial melting of pyroxenites may have significant effects on mantle source compositions and on the physicochemical processes of basalt genesis, while the contribution of pyroxenite-derived melts may not lead to major changes of the compositions of primitive basaltic magmas. The implications of these results for the generation of mid-ocean ridge basalts will be discussed.

Oral Presentation: 19 December
Abstract: V52A-08
Session: Geochemical Heterogeneities in OIB and MORB Sources: Implications for Melting Processes and Mantle Dynamics III
AGU Fall Meeting
San Francisco
15-19 December 2008
The involvement of pyroxenites has often been suggested to solve major problems in basalt petrogenesis (e.g., Hirschmann and Stolper, 1996). Numerous high pressure experiments were devoted to the study of partial melting of these rock-types, and allowed to better constrain their melting relations at pressures > 2 GPa (where garnet and pyroxenes are the principal residual phases). As the melting of pyroxenites can also contribute to basalt petrogenesis at pressures < 2 GPa, we performed partial melting experiments on three natural pyroxenites from Beni Bousera ultramafic massif (Morocco) at 1 and 1.5 GPa in a piston-cylinder apparatus: a garnet clinopyroxenite A, a garnet pyroxenite B, and a websterite C; A is silica-undersaturated, while B and C are silica-saturated. These three rocks correspond to frequency maxima of the compositional cloud of pyroxenites worldwide, and can provide an insight into the links between bulk compositions and melting relations. Run products were characterized by scanning electron microscopy and electron probe microanalysis, and phase proportions were computed by mass balance. Our main results may be summarized as follows:
(1) Despite important differences in their bulk compositions, the three investigated pyroxenites show similar melting relations and melt evolutions at moderate pressures (1-1.5 GPa). In particular, the clinopyroxene (cpx) contribution to the melting reaction increases with increasing temperature (at fixed pressure), and the cpx fraction in residual phase assemblages increases with increasing pressure.
(2) Pyroxenite-derived melts have higher FeO and lower MgO and SiO2 than peridotite-derived melts. Moreover, they are nepheline-normative on the whole melting interval for silica-undersaturated composition A, and up to melting degrees of ≈ 40% for silica-saturated compositions (B and C), suggesting that the production of nepheline-normative primary melts is facilitated at low pressure in the presence of pyroxenitic rock-types in the basalt source regions. Nevertheless, pyroxenite- and peridotite-derived melt compositions show a wide overlap, and the variation ranges of pyroxenite-devived melts for many oxides (Al2O3, CaO, TiO2, Na2O, K2O) are close to that of peridotite-derived melts.
(3) Despite these similarities in melt compositions, we observed important differences in melt productivities and solidus temperatures as a function of bulk composition and pressure. For instance, the large fraction of plagioclase (30 wt%) in B at 1 GPa increases the solidus temperature and the melt productivity. On the contrary, the increase of the cpx fraction in residual assemblages with increasing pressure tends to decrease the melt productivity.
Hence, our results suggest that, independently of their bulk composition, partial melting of pyroxenites at 1-1.5 GPa produces liquids with major element patterns close to those produced by peridotite melting. However, bulk pyroxenite compositions control mineralogical assemblages, that in turn control solidus temperatures and melt productivities, and so the relative contributions of various pyroxenites and peridotites to basalt genesis. The implications of these results for MORB petrogenesis will be discussed.
References:
Hirschmann MM, Stolper EM (1996), Contrib. Mineral. Petrol., 124: 185-208.
* Denotes the speaker

Oral Presentation: 9 September 2008
XIIth International Symposium on Experimental Mineralogy, Petrology and Geochemistry
Insbruck, Austria
7-10 December 2008
The compositions of primitive mid-ocean ridge basalts (MORBs) are not only controlled by partial melting processes at depth, but also by magma-rock interactions en route to the surface (as well as shallow-level fractional crystallization). During their ascent, basalts dissolve pyroxenes in the surrounding peridotites, ultimately leading to the formation of high-porosity dunitic channels. Focused flow in dunitic channels may play a major role in the dynamics of magma transport beneath mid-ocean ridges (Kelemen PB & al., 1995, Nature,375: 747-753).
We performed a series of experiments in a piston-cylinder apparatus to determine the effects of focused magma transport on the composition of the ascending basalt and on the formation of dunitic channels. We assumed that the system follows an adiabatic decompression path and that magma focusing occurs instantaneously at 1.25 GPa: at this pressure, the total mass of liquid in the system is multiplied by a factor , referred to as the focusing factor. We first determined the equilibrium melt composition of fertile mantle MBK at 1.25 GPa-1310°C; this composition was then synthesized as a gel and added in different proportions to peridotite MBK to simulate focusing factors equal to 3 and 6. Peridotite MBK and the two basalt-enriched compositions were equilibrated at 1 GPa-1290°C, 0.75 GPa-1270°C, and 0.5 GPa-1250°C.
Our main result is that, at 0.5 GPa-1250°C and Ω=6, the liquid composition is very close to primitive MORB compositions and is in equilibrium with olivine only. Therefore, this strengthens the hypothesis that magma transport by focused flow can explain both the formation of dunite channels beneath mid-ocean ridges and the fact that MORBs are not in equilibrium with a harzburgitic residue at low pressure.
Poster: 16 April 2007
Geophysical Research Abstracts, Vol. 9, 03387, 2007
SRef-ID: 1607-7962/gra/EGU2007-A-03387
European Geosciences Union, General Assembly
Vienna, Austria
15-20 April 2007
The gravitational load of a volcanic edifice is recognised to cause modifications to the underlying substrate and ductile intrusive complexes, producing lateral motion away from the centre and in turn resulting in the development of structures and deformation fields on the volcano. Previous work has concentrated on laterally homogenous systems, resulting in relatively simple structures, which although clear are not necessarily comparable to real-life settings.We use a series of analogue models to show the structures and deformation fields deriving from variations in both the edifice shape and the weak substrate dimensions.
There are significant differences in the structure and deformation fields of central volcanoes (e.g. Mount Etna; Concepción) compared to “ridge-like” volcanic complexes (e.g. Poas, Kilauea). Single volcanoes with realistic topography have a system of summit grabens and conjugate strike-slip faults at the base, which relay the deformation away from the volcano. “Ridge-like” edifices present a straight deformation front, which forces the formation of basal thrusts, which in turn act as the limit for deformation. The form of structures and deformation on the edifice is also influenced by the slope of the underlying substrate, and may cause a situation where spreading is confined to a sector of the cone, increasing the potential of catastrophic collapse of the volcano flank. We use morpho-structural comparisons with the global SRTM dataset to show that the features developed in the models are present on numerous volcanoes in differing tectonic settings, and as such volcano spreading should be considered one of the dominant modifying processes at volcanoes.
* Denotes the speaker
Poster: 28 April 2005
Geophysical Research Abstract, vol. 7, 03045
SRef-ID: 1607-7962/gra/EGU05-A-03045
European Geosciences Union, General Assembly
Vienna, Austria
24-29 April 2005
Depuis une vingtaine d’années, la connaissance de la variabilité chimique et pétrographique des laves formées en contexte d’accrétion océanique (MORB) s’est considérablement approfondie. Il est ainsi apparu que le manteau supérieur sub-océanique est beaucoup plus hétérogène que ne le laissait penser la relative homogénéité chimique des MORB. Ainsi, le manteau contient une fraction significative de pyroxénites qui pourraient jouer un rôle important dans la production des basaltes. De même, l’importance des interactions entre les magmas en cours d’ascension et leur encaissant péridotitique a longtemps été sous-estimée. Le transport des magmas basaltiques dans des chenaux dunitiques de haute perméabilité formés par la focalisation des magmas en profondeur est le mécanisme généralement proposé sous les rides médio-océaniques, mais l’ampleur des transformations chimiques subies par les magmas au cours ce processus est encore peu connue.
J’ai réalisé des expériences en piston-cylindre entre 0.5 et 2.5 GPa pour caractériser les relations de fusion et les compositions de liquide de trois pyroxénites et pour quantifier les effets des interactions magma/péridotite. La technique d’extraction des liquides par microdike a été utilisée pour obtenir des analyses de verre fiables même à de très faibles degrés de fusion. L’objectif était d’apporter des réponses à trois problèmes importants de la pétrogenèse des MORB : Quel est le rôle des pyroxénites sur la composition en éléments majeurs des MORB ? Quel est le sort des liquides pyroxénitiques durant leur passage à travers le manteau péridotitique ? Quelles sont les transformations induites par la focalisation des magmas et les interactions magma/roche sur la composition chimique des liquides et sur la chimie et la minéralogie de l’encaissant péridotitique ?
Les données acquises au cours de cette thèse constituent une base de données importante sur les relations de fusion des pyroxénites et sur les compositions des liquides issus des pyroxénites, en particulier les compositions aux faibles degrés de fusion. Les expériences de fusion partielle menées sur trois pyroxénites représentatives de la variabilité compositionnelle mondiale montrent que, à 1 et 1.5 GPa, la plupart des liquides issus des pyroxénites présentent des compositions en éléments majeurs très similaires à celles des liquides péridotitiques. Ces liquides sont susceptibles de remonter à la surface en interagissant peu ou pas avec le manteau environnant. A plus haute pression, les interactions entre les liquides issus des pyroxénites et le manteau péridotitique subsolidus aboutissent au contraire à une consommation substantielle des liquides et à une forte cristallisation de clinopyroxènes susceptibles d’isoler les pyroxénites de leur encaissant et de créer de nouvelles hétérogénéités lithologiques dans le manteau supérieur. Par conséquent, la fusion partielle des pyroxénites peut avoir des effets significatifs sur la composition minéralogique et chimique du manteau et sur les dynamiques d’extraction et de transport des magmas. Au contraire, dans la plupart des cas, la contribution de liquides issus des pyroxénites n’entraîne pas de changement majeur dans les compositions des magmas basaltiques primitifs. Plus rarement, les pyroxénites produisent des liquides anormalement pauvres en silice et riches en fer dont on retrouve peut-être la signature dans une classe de MORB primitifs avec FeO > 8.5 % et SiO2 < 48.7 % pds. Enfin, les expériences simulant le processus de focalisation des magmas sous les rides médioocéaniques confirment que la focalisation peut expliquer à la fois la formation de chenaux dunitiques et la composition des MORB primitif, en particulier leur sous-saturation en orthopyroxènes.

Over the last twenty years, the knowlege of MORB petrological and chemical variability has been considerably extended. The sub-oceanic mantle, engaged in the partial melting at the MORB origin, has been revealed as relatively heterogeneous, and containing a significant fraction of pyroxenites that may play an important role in basalt generation. In the same way, the effect of the magma/peridotite interactions has been often underestimated. Usually, the transport of magma into high permeable dunitic channels formed by focused flow is the mechanism proposed beneath mid-ocean ridges, but the chemical transformations of magmas during the process is badly constrained.
In this study, experiments have been conducted in piston-cylinder apparatus between 0.5 and 2.5 GPa in order to characterise melting relationships and the melt compositions of three pyroxenites, and also to quantify the effects of magma/peridotite interactions. The microdike technique was used to separate the liquid from the solid phases and to obtain reliable glass analyses, even at low degrees of melting. The aim was to answer three questions concerning the MORB petrogenesis: What is the role of pyroxenites in the major element composition of MORB? What is the fate of pyroxenite-derived melts into the peridotitic mantle? What transformations are inferred by magma focusing and magma/rock interactions on the melt composition and on the chemical and mineralogical composition of the host peridotite?
This study provides a large database for the melting relationships of pyroxenites and for the pyroxenite-derived melt compositions, especially at low degrees of melting. Partial melting experiments performed on three pyroxenites, representative of worldwide compositional variability, show that at 1 and 1.5 GPa, in many instances and for most major elements, pyroxenite-derived melts are similar to peridotite-derived melts. These melts may undergo minor interactions with the overlying peridotitic mantle. On the contrary, at higher pressure the interactions between pyroxenite-derived melts and the subsolidus peridotitic mantle lead to a substantial consumption of the percolating melt and to a crystallization of clinopyroxenes. This is likely to impede further infiltration and to increase the ultramafic rock diversity in the upper mantle. Hence, the partial melting of pyroxenites may have significant effects on mantle source compositions and on the physicochemical processes of basalt genesis, while the contribution of pyroxenite-derived melts may not lead to major changes of the compositions of primitive basaltic magmas. Less frequently, pyroxenites yield melts with very SiO2-poor and FeO-rich compositions that may explain the major element signature of a class of primitive MORB with FeO > 8.5 wt. % and SiO2 < 48.7 wt. % Experiments on the effects of focused magma transport beneath mid-ocean ridges confirm that magma focusing can explain both the formation of dunite channels beneath mid-ocean ridges and the composition of primitive MORBs, and especially their under-saturation of orthopyroxene.
Le transport des magmas basaltiques sous les rides médio-océaniques se ferait principalement dans des chenaux dunitiques de haute perméabilité résultant de la dissolution complète des pyroxènes dans un encaissant péridotitique. C’est la focalisation de l’écoulement magmatique dans des zones privilégiées du manteau qui aboutirait à la formation de ces chenaux. Une série d’expériences en piston-cylindre a été réalisée afin de simuler le processus de focalisation du magma sous les rides médio-océaniques et d’étudier le rééquilibrage entre péridotite et basalte en fonction de la pression et du rapport magma/roche. Les résultats ont ensuite été comparés aux prévisions du programme pMELTS.
Cette étude nous permet de valider un modèle pour la formation des MORB primitifs aux rides médio-océaniques impliquant : 1) la fusion d’un manteau lherzolitique en profondeur, 2) la focalisation du magma qui entraîne la formation de chenaux dunitiques et une évolution des compositions des liquides vers des compositions de MORB primitifs par interaction avec l’encaissant dunitique. 3) La déstabilisation de ces chenaux de haute perméabilité en filons est envisageable pour de forts facteurs de focalisation. Cette étude confirme donc la nécessité d’invoquer formation de dunites sous les rides médio-océaniques pour expliquer la sous-saturation en opx des MORB à basse pression ; elle renforce l’hypothèse que des chenaux de forte porosité constituent le moyen principal du transport des magmas sous les rides. En outre, ces expériences montrent que la focalisation du magma a une forte influence sur la composition minéralogique du résidu pouvant conduire à la formation d’assemblages dunitiques, wherlitiques et gabbroïques. Enfin, les résultats suggèrent que la composition du liquide joue un rôle important dans la modification des compositions chimiques des résidus.

The transport of basaltic melts beneath oceanic spreading centers occurs predominantly in highporosity dunitic channels, resulting from the complete dissolution of pyroxenes in peridotites mantle. The formation of dunitic assemblages is due to magma focussing that is, to the circulation of important melt volumes in these channels. Many experiments were run in a piston-cylinder apparatus to simulate magma focussing processes beneath oceanic spreading centres, and to study the reaction between peridotites and basalt, as function of pressure and magma:rock ratio. Then, results were compared with pMELTS predictions.
This study proposes a primitive MORBs formation model, implying: 1) partial melting of lherzolitic mantle at depth, 2) magma focussing involving the formation of dunitic channels and liquids evolution toward primitive MORBs composition by interaction with surrounding dunites. 3) Destabilization of high-porosity channels to form dykes is possible at high degrees of melting. Thus, this study confirms the necessity of dunites formation beneath the oceanic ridges and strengthens the hypothesis that high-porosity channels constitute the main mean of magma transport at oceanic spreading centers. Moreover, these experiments show that magma focussing has a strong influence on residue mineralogical composition leading to the formation of dunitic, wherlitic and gabbroic assemblages. At last, results suggest that melt composition has an important role on chemical compositions of residues.
La taille des cristaux dans les roches magmatiques est contrôlée par les cinétiques de croissance, de dissolution et de nucléation.
Le but de ces expériences est de déterminer le comportement du diopside lorsqu’il est soumis à de faibles variations thermiques pour des températures proches de celle du liquidus. Le comportement du diopside dans le système diopside-anorthite-forstérite a été examiné expérimentalement pour des variations thermiques de 10 et de 20°C en réchauffement et en refroidissement.
Les expériences ont été réalisées avec le four à 1 atm. à trempe verticale. Dans un premier temps, des expériences ont été dirigées isothermiquement pour que tous les changements texturaux puissent être attribués au mûrissement d’Ostwald. Les résultats suggèrent que la croissance du diopside est contrôlée par un mécanisme de nucléation bidimensionnelle. Dans un second temps, le système est soumis à une petite variation thermique. Les données montent qu’un refroidissement ou un réchauffement rapide, même faible, implique un fort déséquilibre du système. Ce dernier subit alors des oscillations entre des phases de croissance et nucléation et des phases de dissolution. Ces oscillations voient cependant leur amplitude diminuer et il est probable que pour des longues durées, le système retourne à un état d’équilibre.
Le peu de données ne nous permettent pas de quantifier ces fluctuations. Cependant, les expériences étant maintenues à température constante après l’étape de réchauffement ou de refroidissement, ces résultats laissent penser que dans un environnement naturel, où beaucoup d’autres paramètres entrent en jeux, les hypothèses sur les séquences de cristallisation des roches pourraient être sujet à caution.

No english version of this abstract.
Dr Sarah Lambart
Geology and Geophysics, Frederick Albert Sutton Building
115 S 1460 E, Room 409
Salt Lake City, UT 84112-0102